Abstract
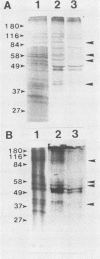
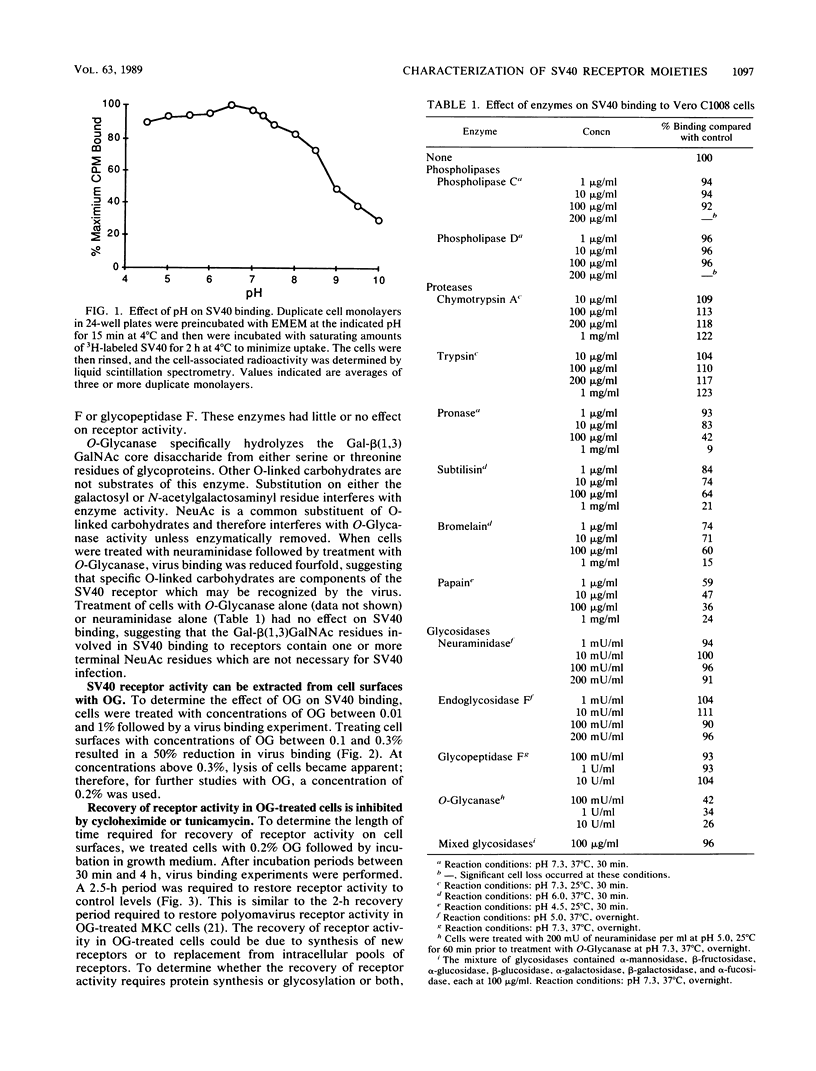
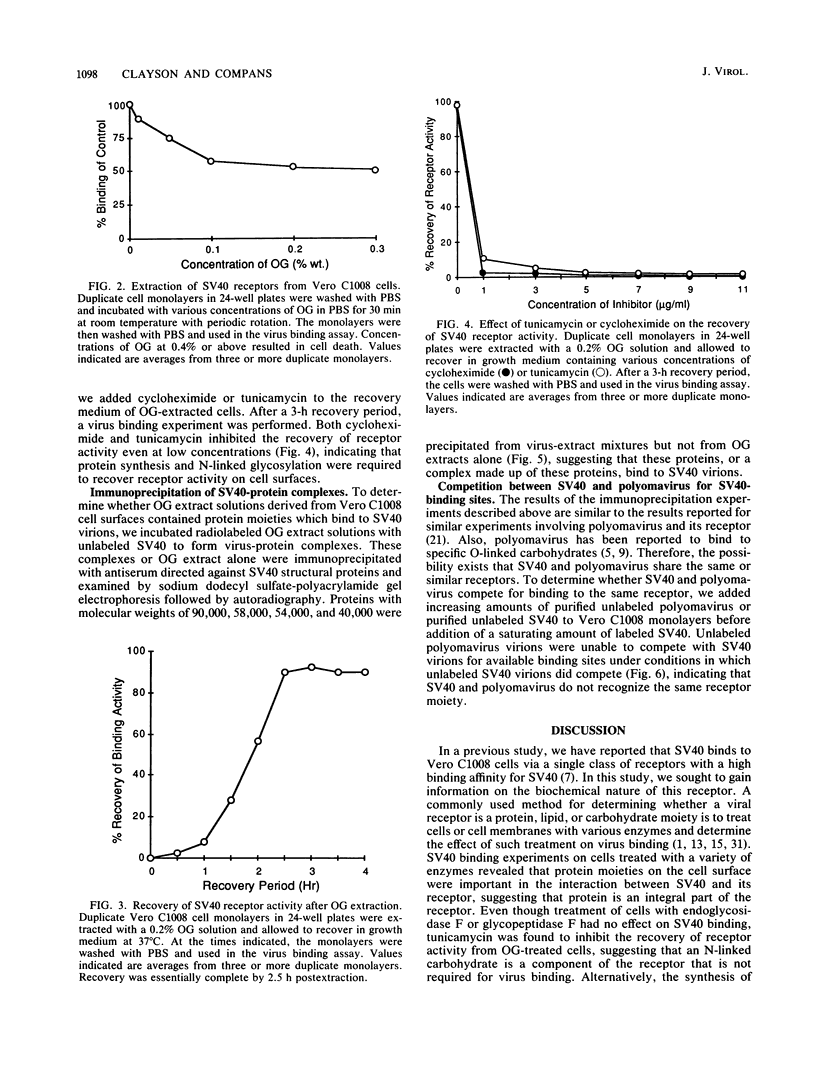
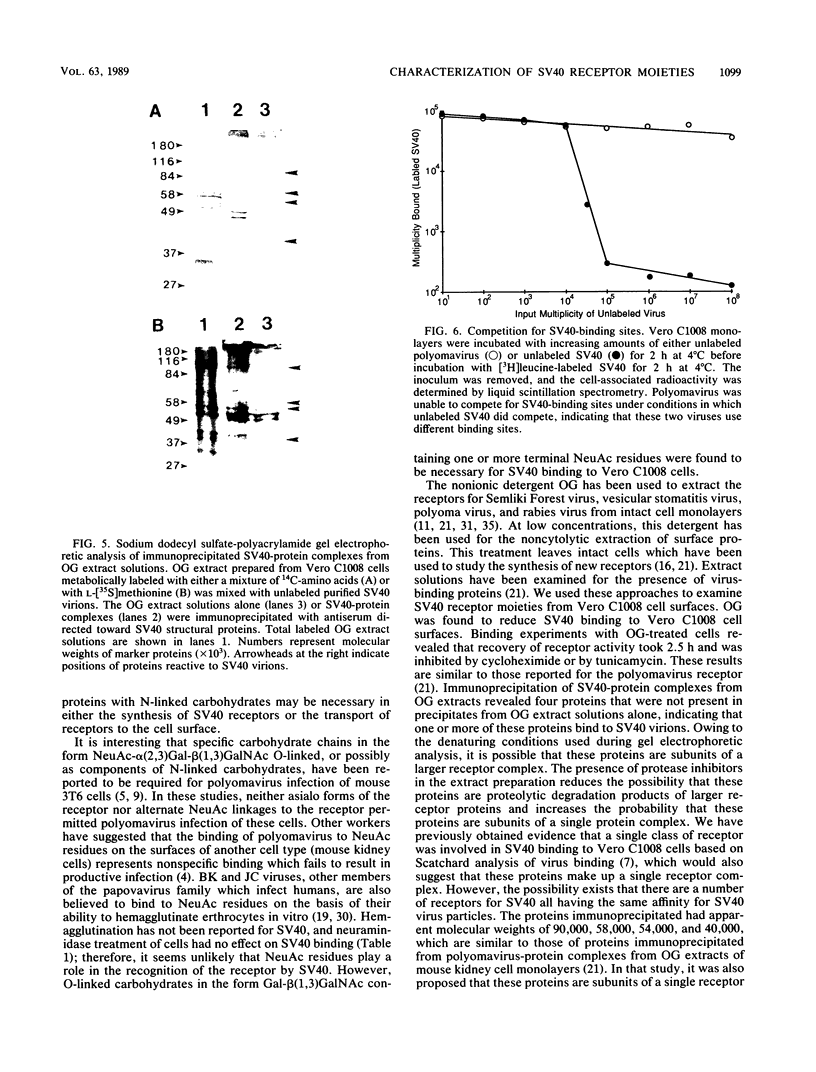
The nature of the simian virus 40 (SV40) receptor on the surfaces of Vero C1008 cells was investigated by a virus binding assay. The optimum pH for SV40 binding to cell surfaces was found to be at 6.5; however, there was little difference in SV40 binding in the range between pH 4.5 and 7.3. The treatment of cell surfaces with several proteases or with an enzyme specific for O-linked carbohydrates significantly reduced virus binding, suggesting that the receptor for SV40 contains protein and O-linked carbohydrates. Treatment of cell monolayers with octyl glucoside removed virus-binding activity from cell surfaces. Recovery of virus-binding activity by octyl glucoside-treated cells took 2.5 h and was inhibited by cycloheximide or tunicamycin. Four polypeptides with molecular weights of 90,000, 58,000, 54,000, and 30,000 were immunoprecipitated from virus-protein complexes derived from octyl glucoside extract solutions and therefore may be components of the SV40 receptor. Competition experiments between SV40 and polyomavirus revealed that these two viruses do not share the same receptor on Vero C1008 cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHKENAZI A., MELNICK J. L. Induced latent infection of monkeys with vacuolating SV-40 Papova virus. Virus in kidneys and urine. Proc Soc Exp Biol Med. 1962 Nov;111:367–372. doi: 10.3181/00379727-111-27794. [DOI] [PubMed] [Google Scholar]
- Armstrong G. D., Paul R. W., Lee P. W. Studies on reovirus receptors of L cells: virus binding characteristics and comparison with reovirus receptors of erythrocytes. Virology. 1984 Oct 15;138(1):37–48. doi: 10.1016/0042-6822(84)90145-4. [DOI] [PubMed] [Google Scholar]
- Barbanti-Brodano G., Swetly P., Koprowski H. Early events in the infection of permissive cells with simian virus 40: adsorption, penetration, and uncoating. J Virol. 1970 Jul;6(1):78–86. doi: 10.1128/jvi.6.1.78-86.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Consigli R. A. Differential adsorption of polyoma virions and capsids to mouse kidney cells and guinea pig erythrocytes. J Virol. 1979 Nov;32(2):679–683. doi: 10.1128/jvi.32.2.679-683.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahan L. D., Singh R., Paulson J. C. Sialyloligosaccharide receptors of binding variants of polyoma virus. Virology. 1983 Oct 30;130(2):281–289. doi: 10.1016/0042-6822(83)90083-1. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clayson E. T., Compans R. W. Entry of simian virus 40 is restricted to apical surfaces of polarized epithelial cells. Mol Cell Biol. 1988 Aug;8(8):3391–3396. doi: 10.1128/mcb.8.8.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S. Early events in cell-animal virus interactions. Bacteriol Rev. 1973 Jun;37(2):103–135. doi: 10.1128/br.37.2.103-135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H., Cahan L. D., Paulson J. C. Polyoma virus recognizes specific sialyligosaccharide receptors on host cells. Virology. 1981 Feb;109(1):188–192. doi: 10.1016/0042-6822(81)90485-2. [DOI] [PubMed] [Google Scholar]
- Griffith G. R., Marriott S. J., Rintoul D. A., Consigli R. A. Early events in polyomavirus infection: fusion of monopinocytotic vesicles containing virions with mouse kidney cell nuclei. Virus Res. 1988 Apr;10(1):41–51. doi: 10.1016/0168-1702(88)90056-1. [DOI] [PubMed] [Google Scholar]
- Helenius A., Morein B., Fries E., Simons K., Robinson P., Schirrmacher V., Terhorst C., Strominger J. L. Human (HLA-A and HLA-B) and murine (H-2K and H-2D) histocompatibility antigens are cell surface receptors for Semliki Forest virus. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3846–3850. doi: 10.1073/pnas.75.8.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Sokol F. Morphological aspects of the uptake of simian virus 40 by permissive cells. J Virol. 1970 Jul;6(1):87–93. doi: 10.1128/jvi.6.1.87-93.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Gallo R. C. Characterization of Rauscher murine leukemia virus envelope glycoprotein receptor in membranes from murine fibroblasts. J Virol. 1978 Dec;28(3):686–696. doi: 10.1128/jvi.28.3.686-696.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M., Helenius A. pH-induced alterations in the fusogenic spike protein of Semliki Forest virus. J Cell Biol. 1985 Dec;101(6):2284–2291. doi: 10.1083/jcb.101.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai K., Kaplan M., Peeples M. E. The Vero cell receptor for the hepatitis B virus small S protein is a sialoglycoprotein. Virology. 1988 Apr;163(2):629–634. doi: 10.1016/0042-6822(88)90306-6. [DOI] [PubMed] [Google Scholar]
- LeGrue S. J., Macek C. M., Kahan B. D. Noncytolytic extraction of murine tumor-specific transplantation antigens with the nonionic detergent octyl-beta-D-glucopyranoside. J Natl Cancer Inst. 1982 Jul;69(1):131–136. [PubMed] [Google Scholar]
- Lenard J., Miller D. K. Uncoating of enveloped viruses. Cell. 1982 Jan;28(1):5–6. doi: 10.1016/0092-8674(82)90368-3. [DOI] [PubMed] [Google Scholar]
- MEYER H. M., Jr, HOPPS H. E., ROGERS N. G., BROOKS B. E., BERNHEIM B. C., JONES W. P., NISALAK A., DOUGLAS R. D. Studies on simian virus 40. J Immunol. 1962 Jun;88:796–806. [PubMed] [Google Scholar]
- Mackay R. L., Consigli R. A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976 Aug;19(2):620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraldi N. M., Barbanti-Brodano G., Portolani M., La Placa M. Ultrastructural aspects of BK virus uptake and replication in human fibroblasts. J Gen Virol. 1975 Apr;27(1):71–80. doi: 10.1099/0022-1317-27-1-71. [DOI] [PubMed] [Google Scholar]
- Marriott S. J., Griffith G. R., Consigli R. A. Octyl-beta-D-glucopyranoside extracts polyomavirus receptor moieties from the surfaces of mouse kidney cells. J Virol. 1987 Feb;61(2):375–382. doi: 10.1128/jvi.61.2.375-382.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott S. J., Roeder D. J., Consigli R. A. Anti-idiotypic antibodies to a polyomavirus monoclonal antibody recognize cell surface components of mouse kidney cells and prevent polyomavirus infection. J Virol. 1987 Sep;61(9):2747–2753. doi: 10.1128/jvi.61.9.2747-2753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984 Feb 15;218(1):1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Wellsteed J., Kern H., Harms E., Helenius A. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5297–5301. doi: 10.1073/pnas.79.17.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Rovera G., Vorbrodt A., Abramczuk J. Membrane fusion as a mechanism of simian virus 40 entry into different cellular compartments. J Virol. 1978 Dec;28(3):936–944. doi: 10.1128/jvi.28.3.936-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Mäntyjärvi R. A., Arstila P. P., Meurman O. H. Hemagglutination by BK virus, a tentative new member of the papovavirus group. Infect Immun. 1972 Nov;6(5):824–828. doi: 10.1128/iai.6.5.824-828.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Kawai N., Kawai M., Notake K., Ichihara I. Fusion of SV40-induced endocytotic vacuoles with the nuclear membrane. Cell Struct Funct. 1986 Jun;11(2):135–141. doi: 10.1247/csf.11.135. [DOI] [PubMed] [Google Scholar]
- Norkin L. C., Einck K. H. Cell killing by Simian virus 40: protective effect of chloroquine. Antimicrob Agents Chemother. 1978 Dec;14(6):930–932. doi: 10.1128/aac.14.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Rogers C. M., Walker D. L. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun. 1977 Feb;15(2):656–662. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Tralka T. S., Willingham M. C., Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell. 1983 Feb;32(2):639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Shimura H., Umeno Y., Kimura G. Effects of inhibitors of the cytoplasmic structures and functions on the early phase of infection of cultured cells with simian virus 40. Virology. 1987 May;158(1):34–43. doi: 10.1016/0042-6822(87)90235-2. [DOI] [PubMed] [Google Scholar]
- Upcroft P. Simian virus 40 infection is not mediated by lysosomal activation. J Gen Virol. 1987 Sep;68(Pt 9):2477–2480. doi: 10.1099/0022-1317-68-9-2477. [DOI] [PubMed] [Google Scholar]