Abstract
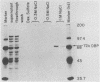
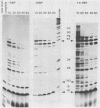
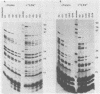
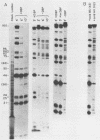
The adenovirus 72-kilodalton DNA-binding protein (DBP) binds to the attenuated RNA derived from the viral major late promoter. Protection from T1 RNase digestion can be observed when DBP is incubated with attenuated RNA. By using attenuated RNA labeled at one end, the T1 RNase digestion pattern can be mapped to residues located at specific sites in this RNA. Heterologous competitor RNAs do not alter the pattern of DBP protection of a labeled attenuated RNA, as does the identical attenuated RNA. These data indicate some specificity of the interaction between DBP and attenuated RNA. Adenovirus infection of monkey cells results in a more efficient attenuation of RNA initiated at the major late promoter and a reduced level of infectious virus. Adenovirus mutations in DBP relieve this restriction. These DBP mutant proteins do not change their binding properties to the attenuated RNA but suggest a mechanism by which DBP plays a role in the adenovirus host range restriction in monkey cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Dreyfuss G. Adenovirus proteins associated with mRNA and hnRNA in infected HeLa cells. J Virol. 1987 Oct;61(10):3276–3283. doi: 10.1128/jvi.61.10.3276-3283.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y., Hay N. Attenuation and modulation of mRNA secondary structure in a feedback control system regulating SV40 gene expression. Mol Biol Rep. 1983 May;9(1-2):91–100. doi: 10.1007/BF00777479. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Hay N. Attenuation may regulate gene expression in animal viruses and cells. CRC Crit Rev Biochem. 1985;18(4):327–383. doi: 10.3109/10409238509086785. [DOI] [PubMed] [Google Scholar]
- Ariga H., Klein H., Levine A. J., Horwitz M. S. A cleavage product of the adenovirus DNA binding protein is active in DNA replication in vitro. Virology. 1980 Feb;101(1):307–310. doi: 10.1016/0042-6822(80)90510-3. [DOI] [PubMed] [Google Scholar]
- Ben-Asher E., Aloni Y. Transcription of minute virus of mice, an autonomous parvovirus, may be regulated by attenuation. J Virol. 1984 Oct;52(1):266–276. doi: 10.1128/jvi.52.1.266-276.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleghon V. G., Klessig D. F. Association of the adenovirus DNA-binding protein with RNA both in vitro and in vivo. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8947–8951. doi: 10.1073/pnas.83.23.8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicker T., Anderson C., Sharp P. A., Sambrook J. Conditional lethal mutants of adenovirus 2-simian virus 40 hybrids. I. Host range mutants of Ad2+ND1. J Virol. 1974 Jun;13(6):1237–1244. doi: 10.1128/jvi.13.6.1237-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N., Skolnik-David H., Aloni Y. Attenuation in the control of SV40 gene expression. Cell. 1982 May;29(1):183–193. doi: 10.1016/0092-8674(82)90102-7. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Temperature-sensitive replication of H5ts125 adenovirus DNA in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4291–4295. doi: 10.1073/pnas.75.9.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. M., Anderson K. P., Klessig D. F. Partial block to transcription of human adenovirus type 2 late genes in abortively infected monkey cells. J Virol. 1985 Nov;56(2):378–385. doi: 10.1128/jvi.56.2.378-385.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H., Maltzman W., Levine A. J. Structure-function relationships of the adenovirus DNA-binding protein. J Biol Chem. 1979 Nov 10;254(21):11051–11060. [PubMed] [Google Scholar]
- Klessig D. F., Grodzicker T. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell. 1979 Aug;17(4):957–966. doi: 10.1016/0092-8674(79)90335-0. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Hassell J. A. Characterization of a variant of human adenovirus type 2 which multiples efficiently in simian cells. J Virol. 1978 Dec;28(3):945–956. doi: 10.1128/jvi.28.3.945-956.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F. Isolation of a variant of human adenovirus serotype 2 that multiplies efficiently on monkey cells. J Virol. 1977 Mar;21(3):1243–1246. doi: 10.1128/jvi.21.3.1243-1246.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Van Schaik F. M., Sussenbach J. S. Nucleotide sequence of the gene encoding adenovirus type 2 DNA binding protein. Nucleic Acids Res. 1982 Aug 11;10(15):4493–4500. doi: 10.1093/nar/10.15.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A., Levine A. J., Anderson S., Osborn M., Rosenwirth B., Weber K. The relationship between group C adenovirus tumor antigen and the adenovirus single-strand DNA-binding protein. Cell. 1976 Apr;7(4):575–584. doi: 10.1016/0092-8674(76)90208-7. [DOI] [PubMed] [Google Scholar]
- Logan J., Nicolas J. C., Topp W. C., Girard M., Shenk T., Levine A. J. Transformation by adenovirus early region 2A temperature-sensitive mutants and their revertants. Virology. 1981 Dec;115(2):419–422. doi: 10.1016/0042-6822(81)90126-4. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok M., Maderious A., Chen-Kiang S. Premature termination by human RNA polymerase II occurs temporally in the adenovirus major late transcriptional unit. Mol Cell Biol. 1984 Oct;4(10):2031–2040. doi: 10.1128/mcb.4.10.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J. C., Ingrand D., Sarnow P., Levine A. J. A mutation in the adenovirus type 5 DNA binding protein that fails to autoregulate the production of the DNA binding protein. Virology. 1982 Oct 30;122(2):481–485. doi: 10.1016/0042-6822(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Nicolas J. C., Sarnow P., Girard M., Levine A. J. Host range temperature-conditional mutants in the adenovirus DNA binding protein are defective in the assembly of infectious virus. Virology. 1983 Apr 15;126(1):228–239. doi: 10.1016/0042-6822(83)90474-9. [DOI] [PubMed] [Google Scholar]
- Nicolas J. C., Suarez F., Levine A. J., Girard M. Temperature-independent revertants of adenovirus H5ts125 and H5ts107 mutants in the DNA binding protein: isolation of a new class of host range temperature conditional revertants. Virology. 1981 Jan 30;108(2):521–524. doi: 10.1016/0042-6822(81)90461-x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer P., Hay N., Pruzan R., Jakobovits E. B., Aloni Y. In vitro premature termination in SV40 late transcription. EMBO J. 1983;2(2):185–191. doi: 10.1002/j.1460-2075.1983.tb01403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnekov O., Ben-Asher E., Bengal E., Choder M., Hay N., Kessler M., Ragimov N., Seiberg M., Skolnik-David H., Aloni Y. Transcription termination in animal viruses and cells. Gene. 1988 Dec 10;72(1-2):91–104. doi: 10.1016/0378-1119(88)90130-8. [DOI] [PubMed] [Google Scholar]
- Seiberg M., Kessler M., Levine A. J., Aloni Y. Human RNA polymerase II can prematurely terminate transcription of the adenovirus type 2 late transcription unit at a precise site that resembles a prokaryotic termination signal. Virus Genes. 1987 Nov;1(1):97–116. doi: 10.1007/BF00125689. [DOI] [PubMed] [Google Scholar]