Abstract
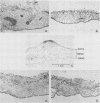
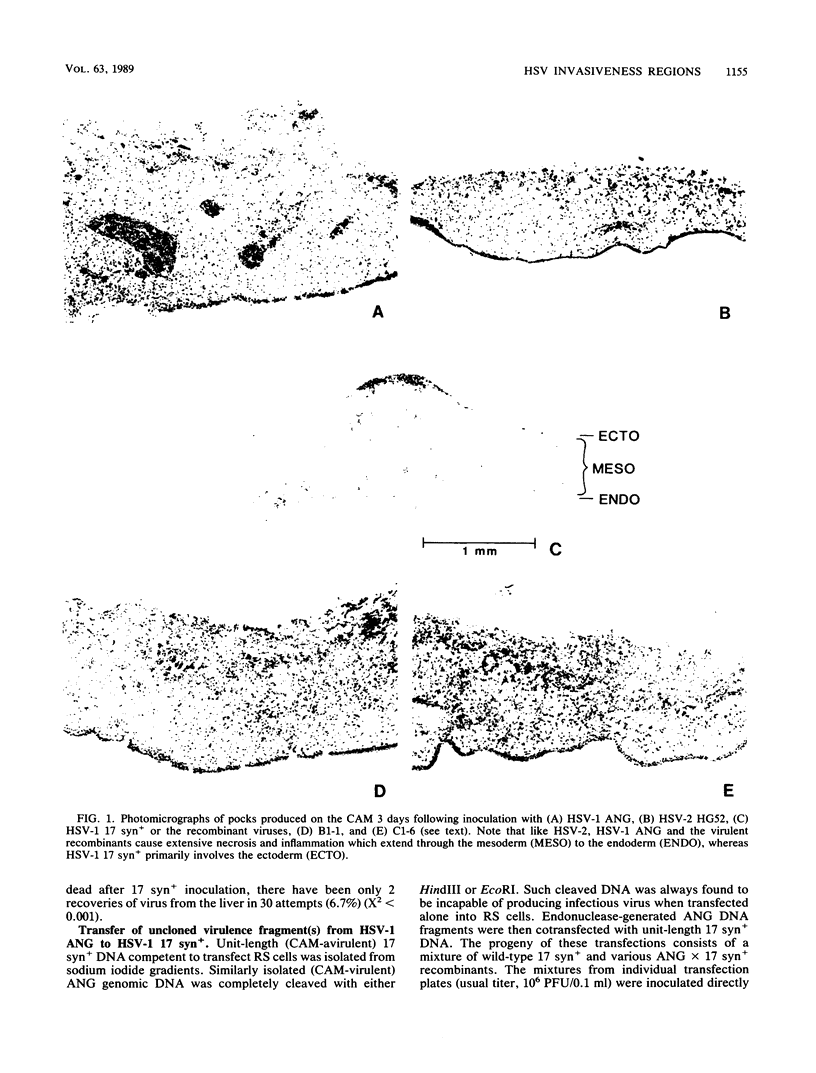
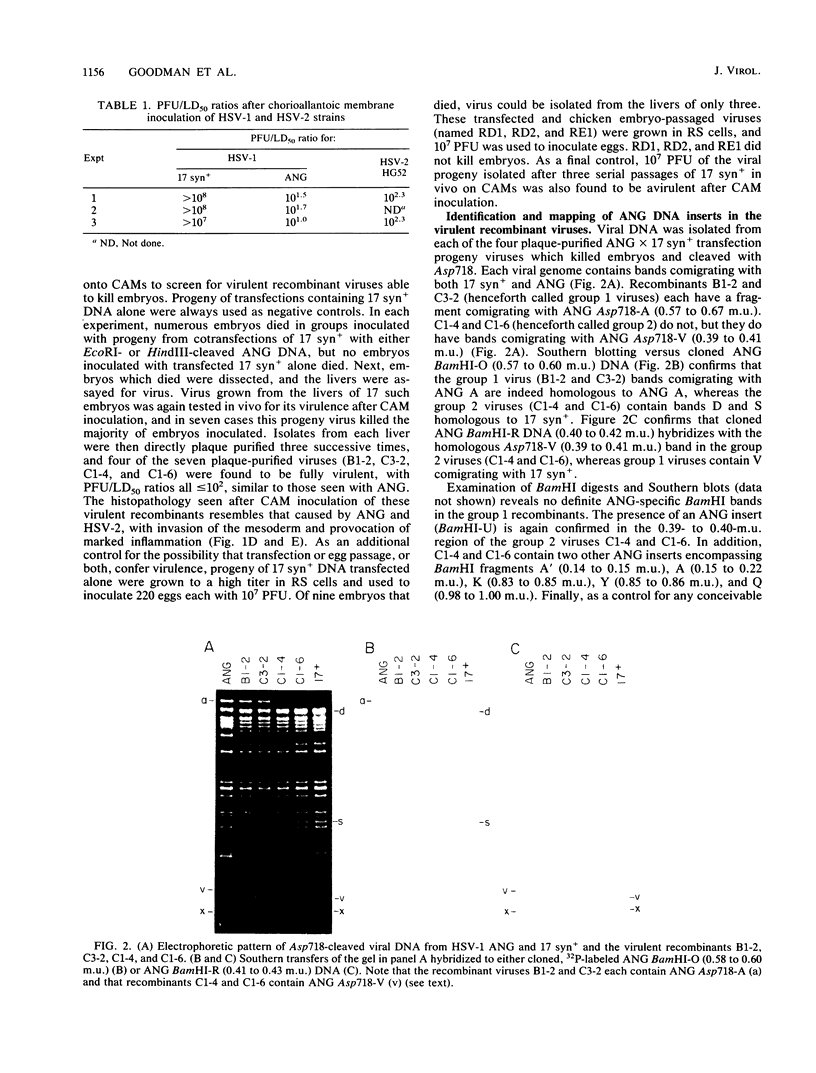
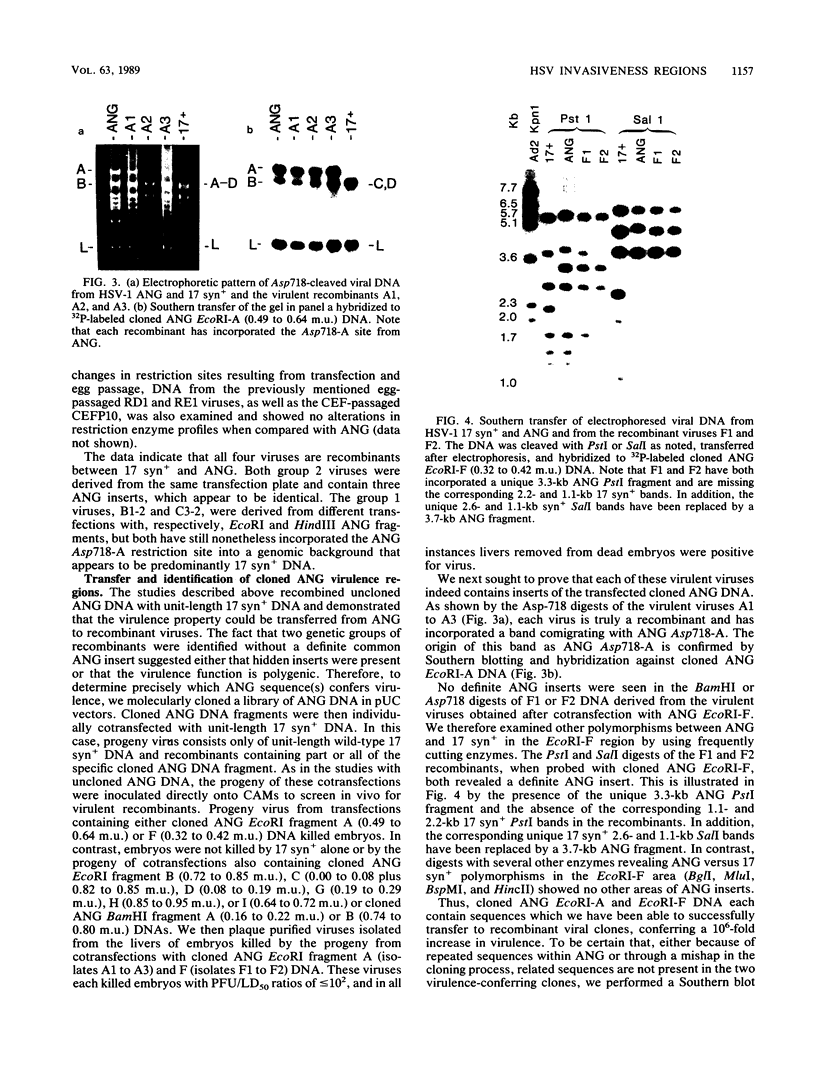
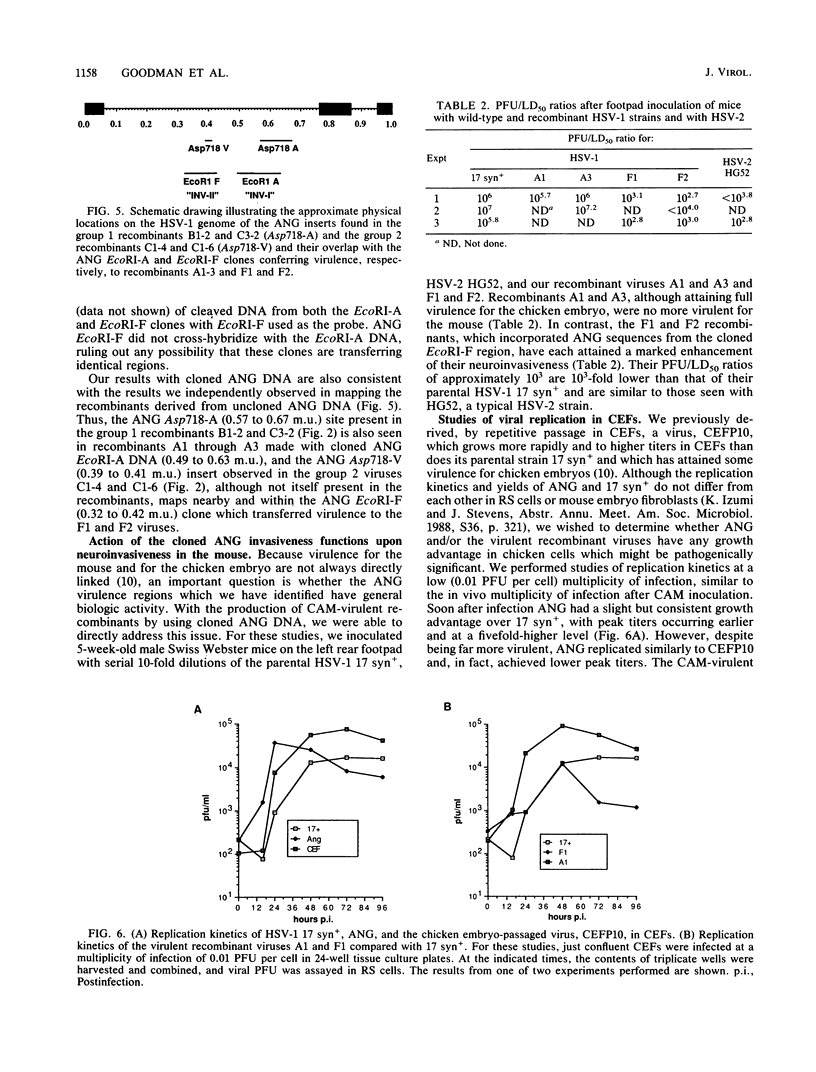
Following peripheral inoculation of experimental animals, herpes simplex virus type 2 (HSV-2) strains are more virulent than HSV-1 strains, and clinical studies suggest that they possess enhanced virulence in humans. One dramatic type-specific difference in virulence is observed following inoculation of the chorioallantoic membrane (CAM) of the chicken embryo: HSV-2, but not HSV-1, makes large pocks on the CAM, invades the mesoderm, generalizes in the embryo, and kills the chicken. These properties have been believed to be specific for HSV-2, and their molecular basis is unknown. We now report that an HSV-1 strain, ANG, behaves even more efficiently than HSV-2. In addition, we have transferred restriction fragments of ANG DNA to another HSV-1 strain, 17 syn+, conferring the CAM virulence phenotype on the normally CAM-avirulent 17 syn+. Like ANG, these recombinant viruses are 10(6)-fold more virulent (PFU/50%) lethal dose [LD50] ratio, less than or equal to 10(2)) than the parental 17 syn+ strain (PFU/LD50 ratio, greater than or equal to 10(8)). A molecularly cloned library of ANG DNA was used to identify two distinct regions containing the virulence functions. Transfer of sequences contained in either cloned ANG EcoRI fragment A (0.49 to 0.64 map units) or F (0.32 to 0.42 map units) DNA to 17 syn+ confers CAM virulence, whereas other cloned regions of the ANG genome do not. Using cloned DNA, we derived and plaque purified several virulent recombinant viruses with inserts from either the ANG EcoRI fragment A (INV-I) or F (INV-II) areas. In each instance, the transfer of the cloned INV-I or INV-II sequences enhanced virulence for the chicken embryo 10(6)-fold (PFU/LD50 ratio, less than or equal to 10(2]. In addition, the transfer of the cloned ANG EcoRI-F INV-II sequences resulted in a 10(3)-fold enhancement of neuroinvasiveness and virulence for mice. Following footpad inoculation, these recombinants kill mice with a PFU/LD50 ratio of approximately 10(3) (similar to HSV-2 strains) compared with 10(6) for 17 syn+. Thus, we have identified, cloned, and transferred two DNA regions from HSV-1 ANG which contain virulence genes (INV-I and INV-II) important in mesodermal invasiveness on the CAM and, in the case of INV-II, neuroinvasiveness in the mouse. In each instance, the recombinant HSV-1 viruses have attained enhanced virulence beyond that described for HSV-1 strains and similar to that seen with HSV-2.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison C., Rixon F. J., Palfreyman J. W., O'Hara M., Preston V. G. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology. 1984 Oct 30;138(2):246–259. doi: 10.1016/0042-6822(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Becker Y., Hadar J., Tabor E., Ben-Hur T., Raibstein I., Rösen A., Darai G. A sequence in HpaI-P fragment of herpes simplex virus-1 DNA determines intraperitoneal virulence in mice. Virology. 1986 Mar;149(2):255–259. doi: 10.1016/0042-6822(86)90128-5. [DOI] [PubMed] [Google Scholar]
- Blacklaws B. A., Nash A. A., Darby G. Specificity of the immune response of mice to herpes simplex virus glycoproteins B and D constitutively expressed on L cell lines. J Gen Virol. 1987 Apr;68(Pt 4):1103–1114. doi: 10.1099/0022-1317-68-4-1103. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- Centifanto-Fitzgerald Y. M., Yamaguchi T., Kaufman H. E., Tognon M., Roizman B. Ocular disease pattern induced by herpes simplex virus is genetically determined by a specific region of viral DNA. J Exp Med. 1982 Feb 1;155(2):475–489. doi: 10.1084/jem.155.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L., Whitley R. J., Stone E. F., Mohan K. Difference between herpes simplex virus type 1 and type 2 neonatal encephalitis in neurological outcome. Lancet. 1988 Jan 2;1(8575-6):1–4. doi: 10.1016/s0140-6736(88)90997-x. [DOI] [PubMed] [Google Scholar]
- Craig C. P., Nahmias A. J. Different patterns of neurologic involvement with herpes simplex virus types 1 and 2: isolation of herpes simplex virus type 2 from the buffy coat of two adults with meningitis. J Infect Dis. 1973 Apr;127(4):365–372. doi: 10.1093/infdis/127.4.365. [DOI] [PubMed] [Google Scholar]
- Dix R. D., McKendall R. R., Baringer J. R. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983 Apr;40(1):103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Preparation and characterization of specific antisera to individual glycoprotein antigens comprising the major glycoprotein region of herpes simplex virus type 1. J Virol. 1980 Sep;35(3):902–917. doi: 10.1128/jvi.35.3.902-917.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. L., Stevens J. G. Passage of herpes simplex virus type 1 on chick embryo fibroblasts confers virulence for chick embryos. Virus Res. 1986 Aug;5(2-3):191–200. doi: 10.1016/0168-1702(86)90017-1. [DOI] [PubMed] [Google Scholar]
- Goodpasture E. W., Woodruff A. M., Buddingh G. J. THE CULTIVATION OF VACCINE AND OTHER VIRUSES IN THE CHORIOALLANTOIC MEMBRANE OF CHICK EMBRYOS. Science. 1931 Oct 9;74(1919):371–372. doi: 10.1126/science.74.1919.371. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Javier R. T., Sedarati F., Stevens J. G. Two avirulent herpes simplex viruses generate lethal recombinants in vivo. Science. 1986 Nov 7;234(4777):746–748. doi: 10.1126/science.3022376. [DOI] [PubMed] [Google Scholar]
- Kaerner H. C., Schröder C. H., Ott-Hartmann A., Kümel G., Kirchner H. Genetic variability of herpes simplex virus: development of a pathogenic variant during passaging of a nonpathogenic herpes simplex virus type 1 virus strain in mouse brain. J Virol. 1983 Apr;46(1):83–93. doi: 10.1128/jvi.46.1.83-93.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty W. E., Coombs R. W., Benedetti J., Critchlow C., Corey L. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N Engl J Med. 1987 Jun 4;316(23):1444–1449. doi: 10.1056/NEJM198706043162304. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Lisle J. J., Darby G. Restoration of wild-type pathogenicity to an attenuated DNA polymerase mutant of herpes simplex virus type 1. J Gen Virol. 1986 Nov;67(Pt 11):2501–2506. doi: 10.1099/0022-1317-67-11-2501. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk K., Ludwig G. Properties of plaque variants of herpes virus hominis strains of genital origin. Arch Gesamte Virusforsch. 1972;37(4):308–315. doi: 10.1007/BF01241453. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Dowdle W. R., Kramer J. H., Luce C. F., Mansour S. C. Antibodies to Herpesvirus hominis types 1 and 2 in the rabbit. J Immunol. 1969 Apr;102(4):956–962. [PubMed] [Google Scholar]
- Nahmias A. J., Dowdle W. R., Naib Z. M., Highsmith A., Harwell R. W., Josey W. E. Relation of pock size on chorioallantoic membrane to antigenic type of herpesvirus hominis. Proc Soc Exp Biol Med. 1968 Apr;127(4):1022–1028. doi: 10.3181/00379727-127-32861. [DOI] [PubMed] [Google Scholar]
- Plummer G., Waner J. L., Bowling C. P. Comparative studies of type 1 and type 2 & 'herpes simplex' viruses. Br J Exp Pathol. 1968 Apr;49(2):202–208. [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Coates J. A., Rixon F. J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983 Mar;45(3):1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers F. G. Infection, haemorrhage and death of chick embryos after inoculation of herpes simplex virus type 2 on to the chorioallantoic membrane. J Gen Virol. 1973 Oct;21:187–191. doi: 10.1099/0022-1317-21-1-187. [DOI] [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979 Mar;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Cook M. L., Devi-Rao G. B., Wagner E. K., Stevens J. G. Functional and molecular analyses of the avirulent wild-type herpes simplex virus type 1 strain KOS. J Virol. 1986 Apr;58(1):203–211. doi: 10.1128/jvi.58.1.203-211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Wagner E. K., Stevens J. G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983 Nov;131(1):180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]