Abstract
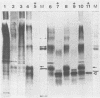
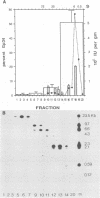
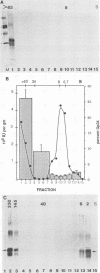
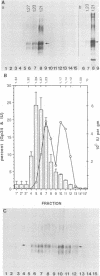
In this report, we present the first physical characterization of the Creutzfeld-Jakob disease agent. Preparations with high yields of infectivity (assayed infectious units) were obtained by a novel, gentle procedure in which initially sedimenting Gp34 ("prion" protein) was disaggregated by a variety of criteria with no subsequent loss of infectivity. Studies with this preparation indicate that most of the Creutzfeld-Jakob disease agent has both a viruslike size and density. In velocity sedimentation and isopycnic sucrose gradients, infectivity comigrated with nucleic acid-protein complexes of appreciable size.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Caughey B., Race R., Vogel M., Buchmeier M., Chesebro B. In vitro expression of cloned PrP cDNA derived from scrapie-infected mouse brain: lack of transmission of scrapie infectivity. Ciba Found Symp. 1988;135:197–208. doi: 10.1002/9780470513613.ch13. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Race R., Wehrly K., Nishio J., Bloom M., Lechner D., Bergstrom S., Robbins K., Mayer L., Keith J. M. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature. 1985 May 23;315(6017):331–333. doi: 10.1038/315331a0. [DOI] [PubMed] [Google Scholar]
- Cho H. J. Is the scrapie agent a virus? Nature. 1976 Jul 29;262(5567):411–412. doi: 10.1038/262411a0. [DOI] [PubMed] [Google Scholar]
- Cho H. J. Requirement of a protein component for scrapie infectivity. Intervirology. 1980;14(3-4):213–216. doi: 10.1159/000149185. [DOI] [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer H., Gelderblom H., Hilmert H., Ozel M., Edelbluth C., Kimberlin R. H. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983 Dec 1;306(5942):476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- Diringer H., Kimberlin R. H. Infectious scrapie agent is apparently not as small as recent claims suggest. Biosci Rep. 1983 Jun;3(6):563–568. doi: 10.1007/BF01120701. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Keller D. S., Helenius A., Balch W. E. Role for adenosine triphosphate in regulating the assembly and transport of vesicular stomatitis virus G protein trimers. J Cell Biol. 1987 Nov;105(5):1957–1969. doi: 10.1083/jcb.105.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Prusiner S. B. Purified prion proteins and scrapie infectivity copartition into liposomes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4017–4021. doi: 10.1073/pnas.84.12.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Gibbs C. J., Jr Search for infectious etiology in chronic and subacute degenerative diseases of the central nervous system. Curr Top Microbiol Immunol. 1967;40:44–58. doi: 10.1007/978-3-642-46059-3_6. [DOI] [PubMed] [Google Scholar]
- Hope J., Morton L. J., Farquhar C. F., Multhaup G., Beyreuther K., Kimberlin R. H. The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J. 1986 Oct;5(10):2591–2597. doi: 10.1002/j.1460-2075.1986.tb04539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska J. F., Robinson W. S. The proteins of hepatitis B Dane particle cores. J Med Virol. 1977;1(2):119–131. doi: 10.1002/jmv.1890010205. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Millson G. C., Hunter G. D. An experimental examination of the scrapie agent in cell membrane mixtures. 3. Studies of the operational size. J Comp Pathol. 1971 Jul;81(3):383–391. doi: 10.1016/0021-9975(71)90026-0. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. A. Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J Gen Virol. 1986 Feb;67(Pt 2):255–263. doi: 10.1099/0022-1317-67-2-255. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Simons K., von Bonsdorff C. H. Studies in subviral components of Semliki Forest virus. Ann Med Exp Biol Fenn. 1969;47(4):235–248. [PubMed] [Google Scholar]
- Lax A. J., Millson G. C., Manning E. J. Involvement of protein in scrapie agent infectivity. Res Vet Sci. 1983 Mar;34(2):155–158. [PubMed] [Google Scholar]
- Liao Y. C., Lebo R. V., Clawson G. A., Smuckler E. A. Human prion protein cDNA: molecular cloning, chromosomal mapping, and biological implications. Science. 1986 Jul 18;233(4761):364–367. doi: 10.1126/science.3014653. [DOI] [PubMed] [Google Scholar]
- Malone T. G., Marsh R. F., Hanson R. P., Semancik J. S. Evidence for the low molecular weight nature of scrapie agent. Nature. 1979 Apr 5;278(5704):575–576. doi: 10.1038/278575a0. [DOI] [PubMed] [Google Scholar]
- Manuelidis E. E., Kim J., Angelo J. N., Manuelidis L. Serial propagation of Creutzfeldt-Jakob disease in guinea pigs. Proc Natl Acad Sci U S A. 1976 Jan;73(1):223–227. doi: 10.1073/pnas.73.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Borden J. Reproducible compartmentalization of individual chromosome domains in human CNS cells revealed by in situ hybridization and three-dimensional reconstruction. Chromosoma. 1988;96(6):397–410. doi: 10.1007/BF00303033. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Manuelidis E. E. Recent developments in scrapie and Creutzfeldt-Jakob disease. Prog Med Virol. 1986;33:78–98. [PubMed] [Google Scholar]
- Manuelidis L., Murdoch G., Manuelidis E. E. Potential involvement of retroviral elements in human dementias. Ciba Found Symp. 1988;135:117–134. doi: 10.1002/9780470513613.ch8. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Sklaviadis T., Manuelidis E. E. Evidence suggesting that PrP is not the infectious agent in Creutzfeldt-Jakob disease. EMBO J. 1987 Feb;6(2):341–347. doi: 10.1002/j.1460-2075.1987.tb04760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Valley S., Manuelidis E. E. Specific proteins associated with Creutzfeldt-Jakob disease and scrapie share antigenic and carbohydrate determinants. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4263–4267. doi: 10.1073/pnas.82.12.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciani D. J., Kuff E. L. Isolation and partial characterization of the internal structural proteins from murine intracisternal A particles. Biochemistry. 1973 Dec 4;12(25):5075–5083. doi: 10.1021/bi00749a008. [DOI] [PubMed] [Google Scholar]
- McCarty K. S., Stafford D., Brown O. Resolution and fractionation of macromolecules by isokinetic sucrose density gradient sedimentation. Anal Biochem. 1968 Aug;24(2):314–329. doi: 10.1016/0003-2697(68)90185-1. [DOI] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathol. 1981;54(1):63–74. doi: 10.1007/BF00691333. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Manuelidis L., Manuelidis E. E. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983 Dec 1;306(5942):474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Pocchiari M., Macchi G., Peano S., Conz A. Can potential hazard of Creutzfeldt-Jakob disease infectivity be reduced in the production of human growth hormone? Inactivation experiments with the 263K strain of scrapie. Rapid communication. Arch Virol. 1988;98(1-2):131–135. doi: 10.1007/BF01321014. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bildstein C., Masiarz F. R., McKinley M. P., Cochran S. P. Electrophoretic properties of the scrapie agent in agarose gels. Proc Natl Acad Sci U S A. 1980 May;77(5):2984–2988. doi: 10.1073/pnas.77.5.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Prions causing degenerative neurological diseases. Annu Rev Med. 1987;38:381–398. doi: 10.1146/annurev.me.38.020187.002121. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Rifkin D. B., Compans R. W. Isolation and characterization of ribonucleoprotein substructures from Rous sarcoma virus. Virology. 1972 Oct;50(1):65–75. doi: 10.1016/0042-6822(72)90346-7. [DOI] [PubMed] [Google Scholar]
- Rizzetto M., Hoyer B., Canese M. G., Shih J. W., Purcell R. H., Gerin J. L. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6124–6128. doi: 10.1073/pnas.77.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis N. K., Sawh P. R., Wolfe G. C., Rubenstein R., Carp R. I., Innis M. A. Isolation of a cDNA clone encoding the leader peptide of prion protein and expression of the homologous gene in various tissues. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6377–6381. doi: 10.1073/pnas.83.17.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siakotos A. N., Gajdusek D. C., Gibbs C. J., Jr, Traub R. D., Bucana C. Partial purification of the scrapie agent from mouse brain by pressure disruption and zonal centrifugation in sucrose-sodium chloride gradients. Virology. 1976 Mar;70(1):230–237. doi: 10.1016/0042-6822(76)90261-0. [DOI] [PubMed] [Google Scholar]
- Sklaviadis T., Manuelidis L., Manuelidis E. E. Characterization of major peptides in Creutzfeldt-Jakob disease and scrapie. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6146–6150. doi: 10.1073/pnas.83.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg K. Surface-active agents for isolation of the core component of avian myeloblastosis virus. J Virol. 1972 Apr;9(4):684–697. doi: 10.1128/jvi.9.4.684-697.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau C., Romet-Lemonne J. L., Mullins J. I., Essex M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell. 1986 Oct 10;47(1):37–47. doi: 10.1016/0092-8674(86)90364-8. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto Y. A., Cardiff R. D., Lund J. K. The structure of the mouse mammary tumor virus: isolation and characterization of the core. Virology. 1977 Mar;77(1):135–148. doi: 10.1016/0042-6822(77)90413-5. [DOI] [PubMed] [Google Scholar]
- Wang K. S., Choo Q. L., Weiner A. J., Ou J. H., Najarian R. C., Thayer R. M., Mullenbach G. T., Denniston K. J., Gerin J. L., Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986 Oct 9;323(6088):508–514. doi: 10.1038/323508a0. [DOI] [PubMed] [Google Scholar]