Abstract
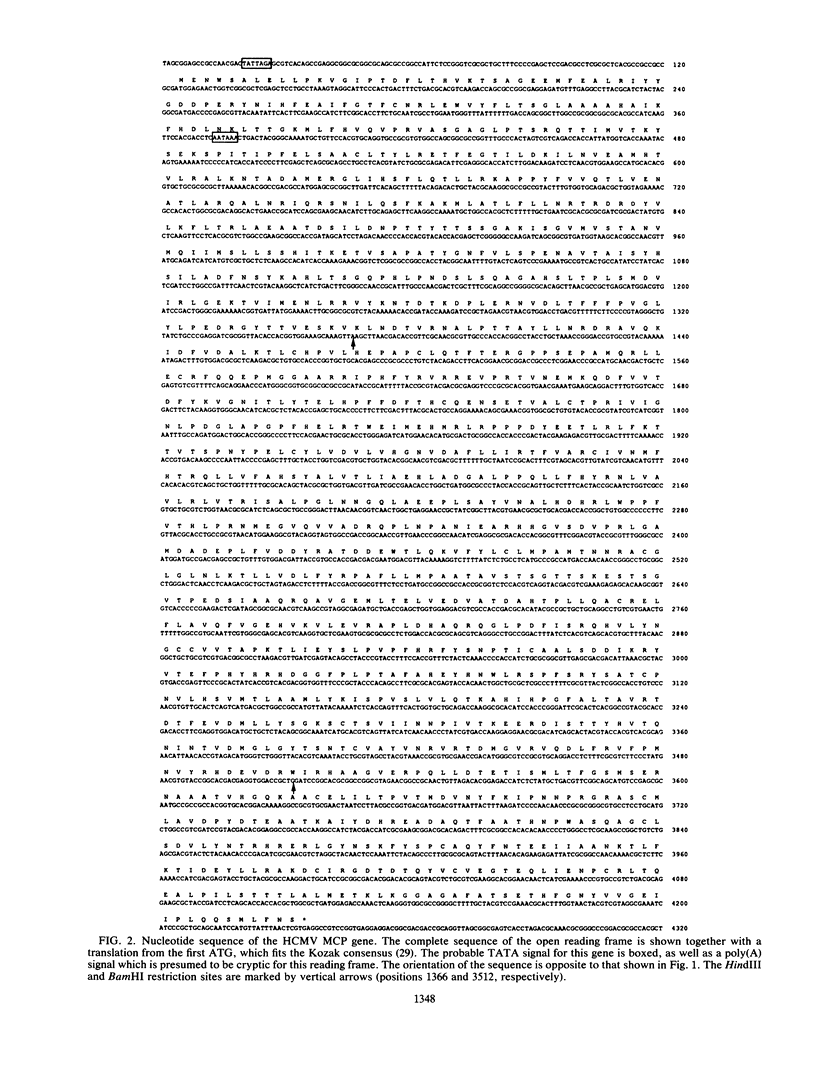
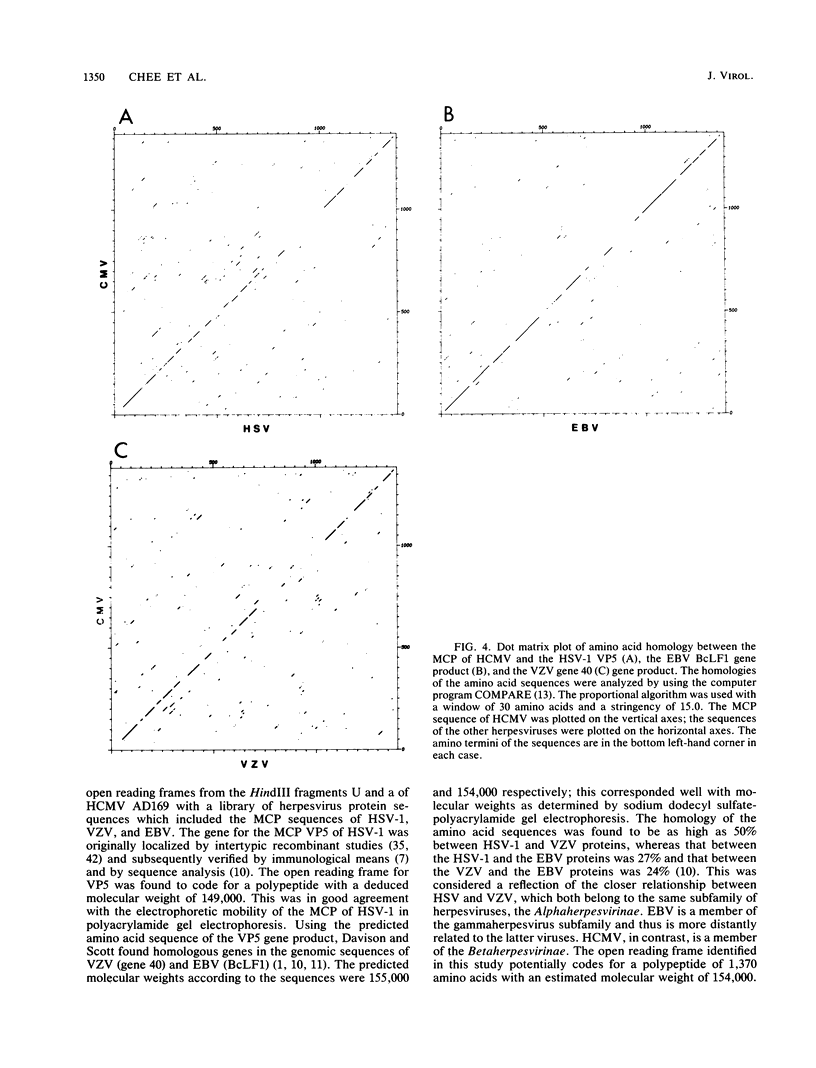
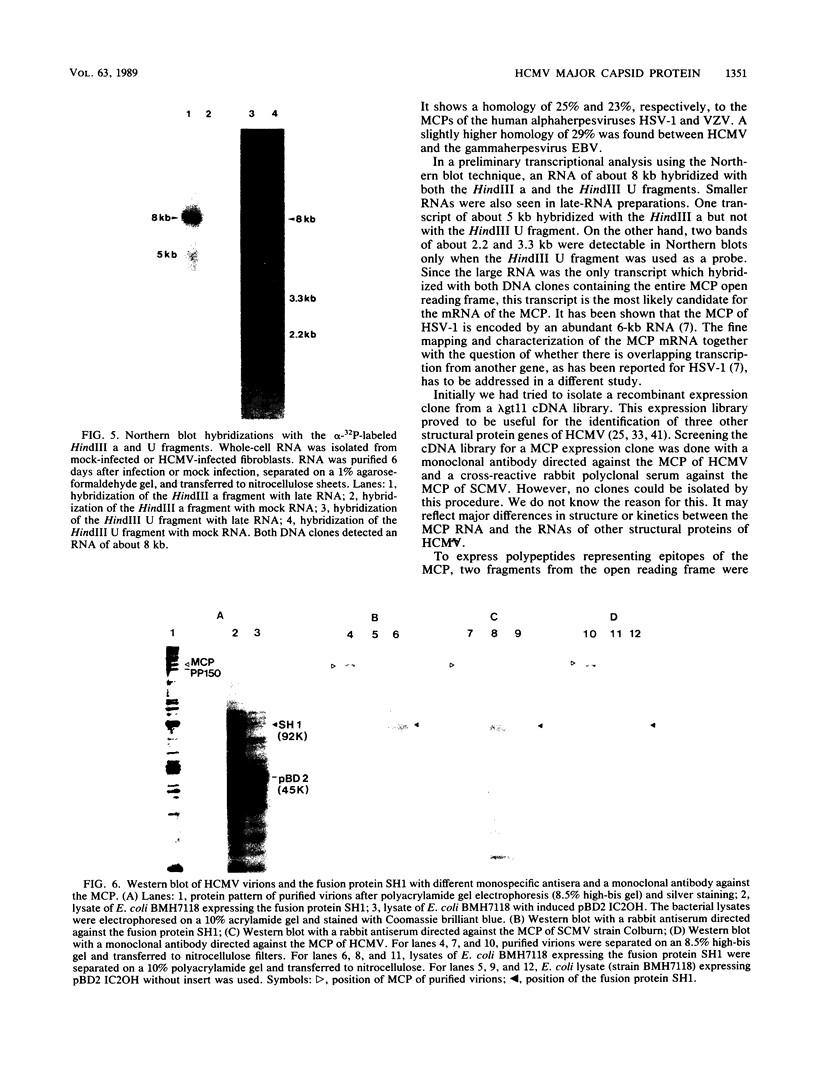
The coding region for the major capsid protein (MCP) of human cytomegalovirus (HCMV) was identified by comparing the protein sequence with the respective sequences of herpes simplex virus (HSV), Epstein-Barr virus, and varicella-zoster virus. The predicted length of the HCMV MCP was 1,370 amino acids. Comparison of the MCP sequences of the different human herpesviruses showed a homology of 25% to the MCP of HSV type 1, a homology of 29% to the MCP of Epstein-Barr virus, and a homology of 23% to the MCP of varicella-zoster virus. A subfragment of the HSV type 1 KpnI i fragment encoding the MCP VP5 cross-hybridized with the HCMV HindIII U fragment containing part of the MCP gene. Northern (RNA) blot analyses with subclones out of the coding region for the HCMV MCP detected one large transcript of about 8 kilobases. A portion of the open reading frame was expressed in Escherichia coli plasmid pBD2 IC2OH as a beta-galactosidase fusion protein and was used to generate polyclonal antibodies in New Zealand White rabbits. The obtained antisera reacted in Western immunoblots with the MCP of purified HCMV virions. A monoclonal antibody against the human MCP and a monospecific rabbit antiserum against strain Colburn of simian cytomegalovirus detected the fusion protein as well as the MCP of purified virions in immunoblots.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Balachandran N., Oba D. E., Hutt-Fletcher L. M. Antigenic cross-reactions among herpes simplex virus types 1 and 2, Epstein-Barr virus, and cytomegalovirus. J Virol. 1987 Apr;61(4):1125–1135. doi: 10.1128/jvi.61.4.1125-1135.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
- Barton G. J., Sternberg M. J. A strategy for the rapid multiple alignment of protein sequences. Confidence levels from tertiary structure comparisons. J Mol Biol. 1987 Nov 20;198(2):327–337. doi: 10.1016/0022-2836(87)90316-0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Cohen G., Eisenberg R., Long D., Wagner E. Direct demonstration that the abundant 6-kilobase herpes simplex virus type 1 mRNA mapping between 0.23 and 0.27 map units encodes the major capsid protein VP5. J Virol. 1984 Jan;49(1):287–292. doi: 10.1128/jvi.49.1.287-292.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Smith G. L., Bell S. E., Hart H., Brown C., Bankier A. T., Tomlinson P., Barrell B. G., Minson T. C. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J Virol. 1988 Apr;62(4):1416–1422. doi: 10.1128/jvi.62.4.1416-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. DNA sequence of the major capsid protein gene of herpes simplex virus type 1. J Gen Virol. 1986 Oct;67(Pt 10):2279–2286. doi: 10.1099/0022-1317-67-10-2279. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Taylor P. Genetic relations between varicella-zoster virus and Epstein-Barr virus. J Gen Virol. 1987 Apr;68(Pt 4):1067–1079. doi: 10.1099/0022-1317-68-4-1067. [DOI] [PubMed] [Google Scholar]
- De Blas A. L., Cherwinski H. M. Detection of antigens on nitrocellulose paper immunoblots with monoclonal antibodies. Anal Biochem. 1983 Aug;133(1):214–219. doi: 10.1016/0003-2697(83)90245-2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Mou S. W. Relative titers of antibodies to individual polypeptide antigens of herpes simplex virus type 1 in human sera. J Infect Dis. 1983 Sep;148(3):436–444. doi: 10.1093/infdis/148.3.436. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Müller I., Collins J. Cloning of the complete human cytomegalovirus genome in cosmids. Gene. 1982 Apr;18(1):39–46. doi: 10.1016/0378-1119(82)90054-3. [DOI] [PubMed] [Google Scholar]
- Geballe A. P., Leach F. S., Mocarski E. S. Regulation of cytomegalovirus late gene expression: gamma genes are controlled by posttranscriptional events. J Virol. 1986 Mar;57(3):864–874. doi: 10.1128/jvi.57.3.864-874.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geballe A. P., Spaete R. R., Mocarski E. S. A cis-acting element within the 5' leader of a cytomegalovirus beta transcript determines kinetic class. Cell. 1986 Sep 12;46(6):865–872. doi: 10.1016/0092-8674(86)90068-1. [DOI] [PubMed] [Google Scholar]
- Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983 Jul 30;128(2):391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- Goins W. F., Stinski M. F. Expression of a human cytomegalovirus late gene is posttranscriptionally regulated by a 3'-end-processing event occurring exclusively late after infection. Mol Cell Biol. 1986 Dec;6(12):4202–4213. doi: 10.1128/mcb.6.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretch D. R., Gehrz R. C., Stinski M. F. Characterization of a human cytomegalovirus glycoprotein complex (gcI). J Gen Virol. 1988 Jun;69(Pt 6):1205–1215. doi: 10.1099/0022-1317-69-6-1205. [DOI] [PubMed] [Google Scholar]
- Gretch D. R., Kari B., Gehrz R. C., Stinski M. F. A multigene family encodes the human cytomegalovirus glycoprotein complex gcII (gp47-52 complex). J Virol. 1988 Jun;62(6):1956–1962. doi: 10.1128/jvi.62.6.1956-1962.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn R., Jahn G., Bürkle A., Freese U. K., Fleckenstein B., zur Hausen H. Genomic localization, sequence analysis, and transcription of the putative human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):119–124. doi: 10.1128/jvi.61.1.119-124.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmiere A., Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983 Oct 15;130(1):118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- Jahn G., Kouzarides T., Mach M., Scholl B. C., Plachter B., Traupe B., Preddie E., Satchwell S. C., Fleckenstein B., Barrell B. G. Map position and nucleotide sequence of the gene for the large structural phosphoprotein of human cytomegalovirus. J Virol. 1987 May;61(5):1358–1367. doi: 10.1128/jvi.61.5.1358-1367.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Scholl B. C., Traupe B., Fleckenstein B. The two major structural phosphoproteins (pp65 and pp150) of human cytomegalovirus and their antigenic properties. J Gen Virol. 1987 May;68(Pt 5):1327–1337. doi: 10.1099/0022-1317-68-5-1327. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Sapienza V. J., Carp R. I., Moon H. M. Analysis of structural polypeptides of purified human cytomegalovirus. J Virol. 1976 Dec;20(3):604–611. doi: 10.1128/jvi.20.3.604-611.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Large-scale rearrangement of homologous regions in the genomes of HCMV and EBV. Virology. 1987 Apr;157(2):397–413. doi: 10.1016/0042-6822(87)90282-0. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mach M., Utz U., Fleckenstein B. Mapping of the major glycoprotein gene of human cytomegalovirus. J Gen Virol. 1986 Jul;67(Pt 7):1461–1467. doi: 10.1099/0022-1317-67-7-1461. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Meyer H., Bankier A. T., Landini M. P., Brown C. M., Barrell B. G., Rüger B., Mach M. Identification and procaryotic expression of the gene coding for the highly immunogenic 28-kilodalton structural phosphoprotein (pp28) of human cytomegalovirus. J Virol. 1988 Jul;62(7):2243–2250. doi: 10.1128/jvi.62.7.2243-2250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak B., Sullivan C., Sarnow P., Thomas R., Bricout F., Nicolas J. C., Fleckenstein B., Levine A. J. Characterization of monoclonal antibodies and polyclonal immune sera directed against human cytomegalovirus virion proteins. Virology. 1984 Jan 30;132(2):325–338. doi: 10.1016/0042-6822(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Downing R. G., Akrigg A., Dollery A. A., Duggleby C. J., Wilkinson G. W., Greenaway P. J. Use of recombinant plasmids to investigate the structure of the human cytomegalovirus genome. J Gen Virol. 1982 Mar;59(Pt 1):111–129. doi: 10.1099/0022-1317-59-1-111. [DOI] [PubMed] [Google Scholar]
- Roby C., Gibson W. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J Virol. 1986 Sep;59(3):714–727. doi: 10.1128/jvi.59.3.714-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüger B., Klages S., Walla B., Albrecht J., Fleckenstein B., Tomlinson P., Barrell B. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J Virol. 1987 Feb;61(2):446–453. doi: 10.1128/jvi.61.2.446-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B. C., Von Hintzenstern J., Borisch B., Traupe B., Bröker M., Jahn G. Prokaryotic expression of immunogenic polypeptides of the large phosphoprotein (pp150) of human cytomegalovirus. J Gen Virol. 1988 Jun;69(Pt 6):1195–1204. doi: 10.1099/0022-1317-69-6-1195. [DOI] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976 Aug;19(2):594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P., Almeida J. D. Human cytomegalovirus: purification of enveloped virions and dense bodies. J Gen Virol. 1977 Aug;36(2):345–349. doi: 10.1099/0022-1317-36-2-345. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner D., Gibson W. Identification of a primate cytomegalovirus group-common protein antigen. Virology. 1981 Nov;115(1):182–191. doi: 10.1016/0042-6822(81)90100-8. [DOI] [PubMed] [Google Scholar]