Abstract
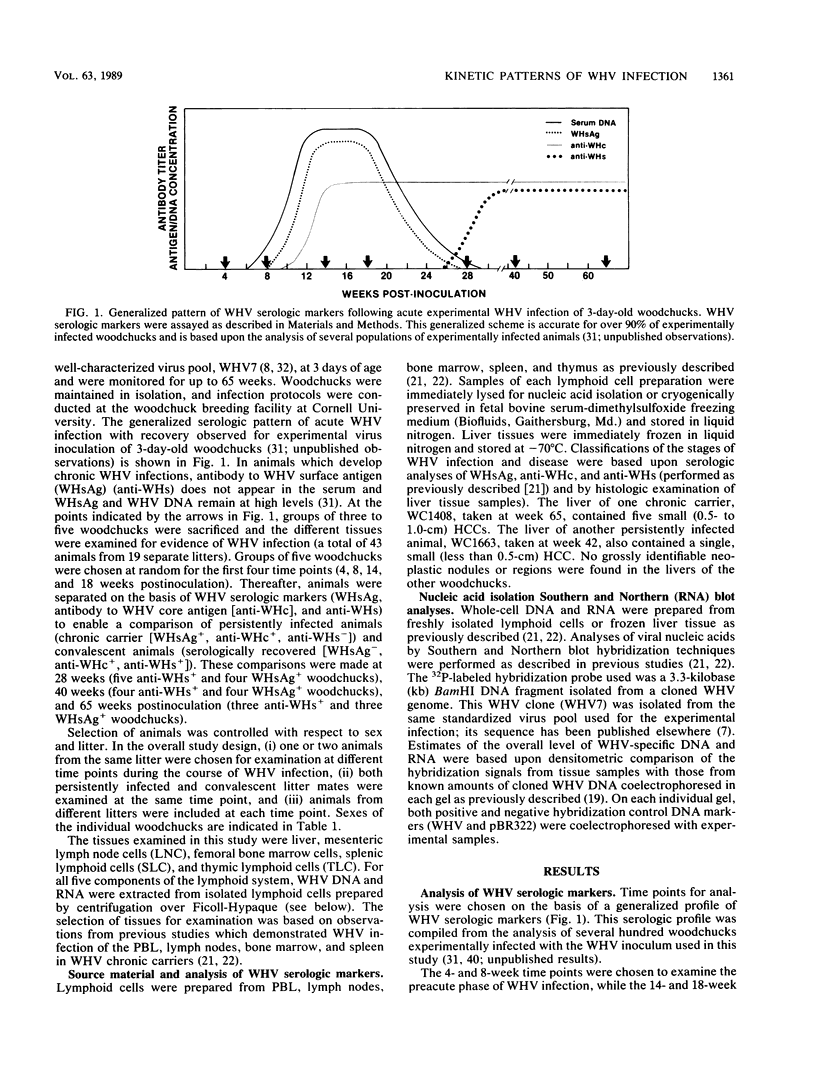
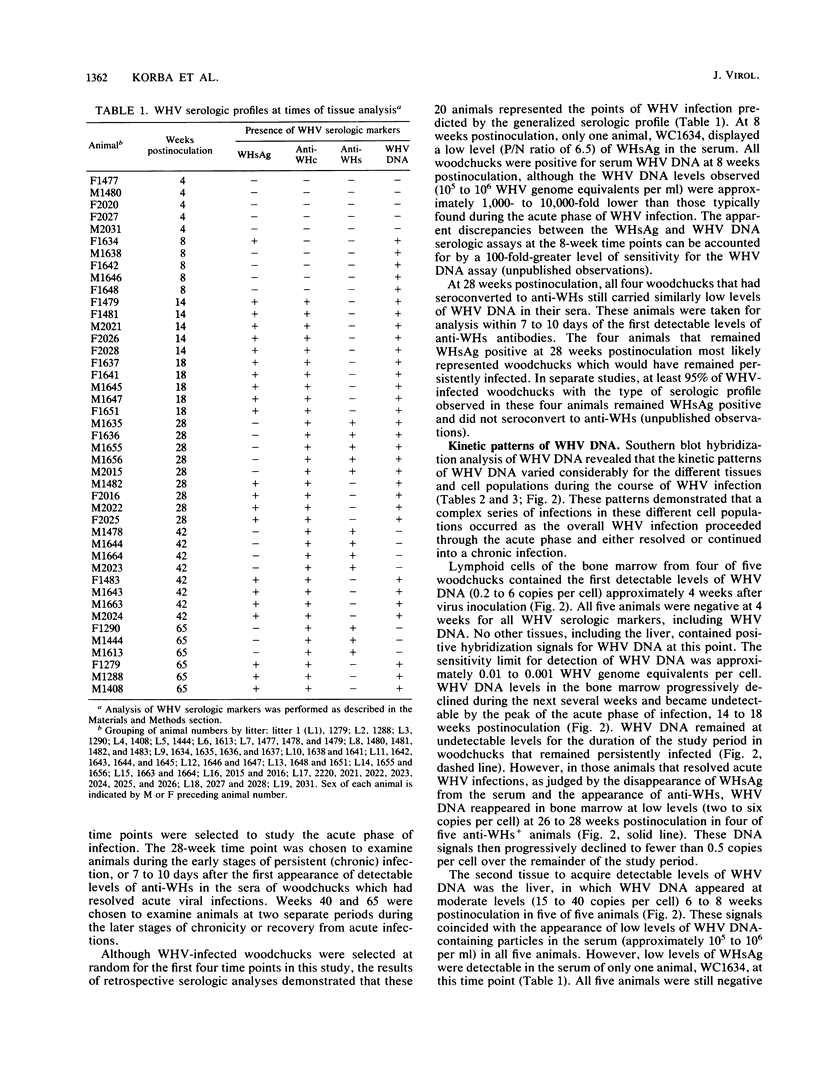
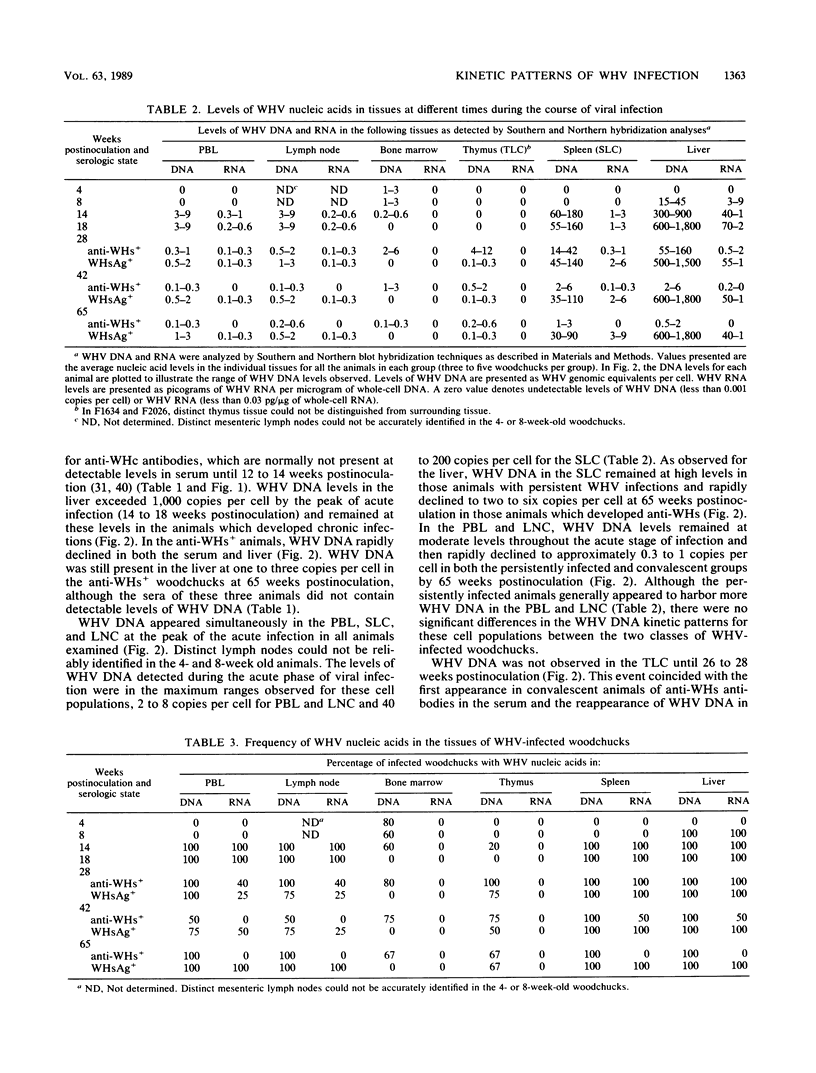
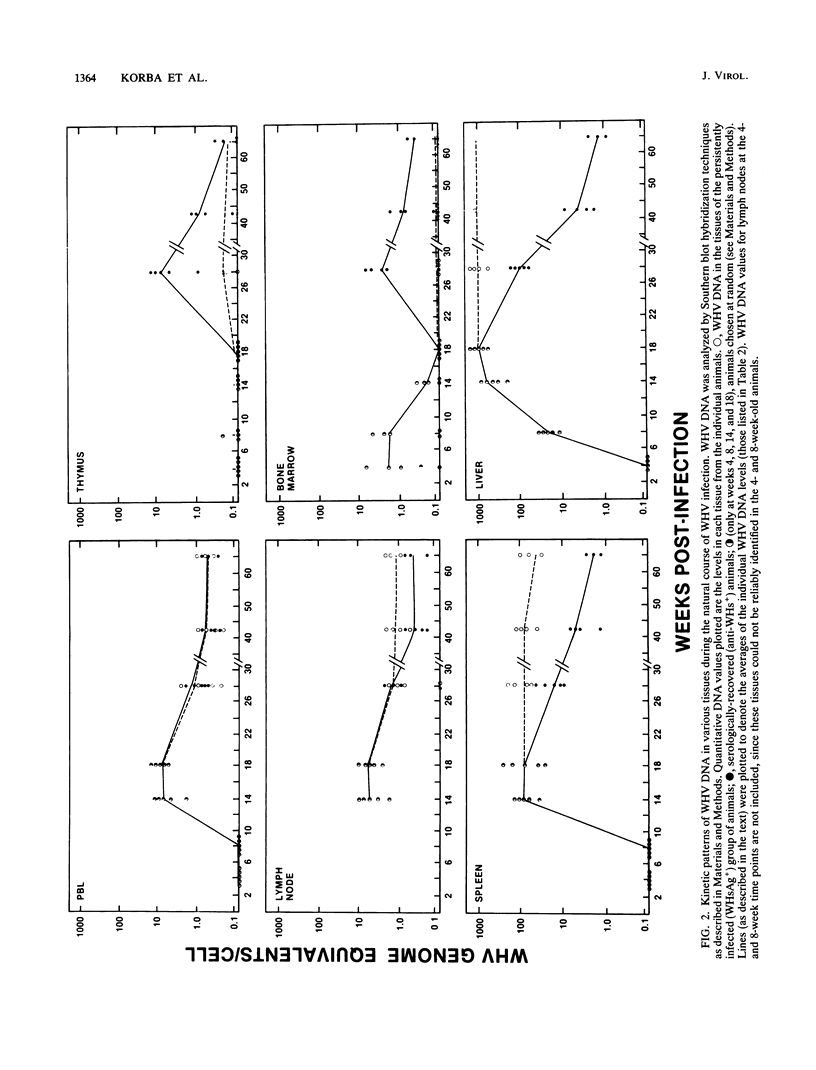
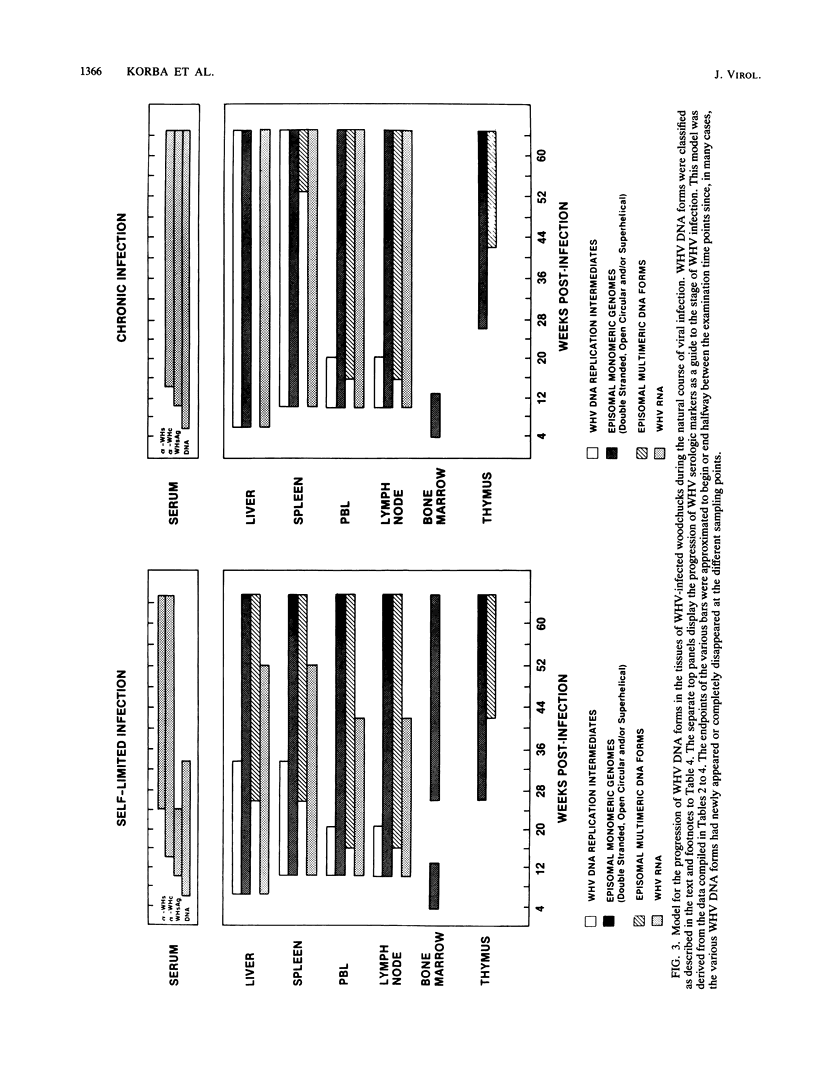
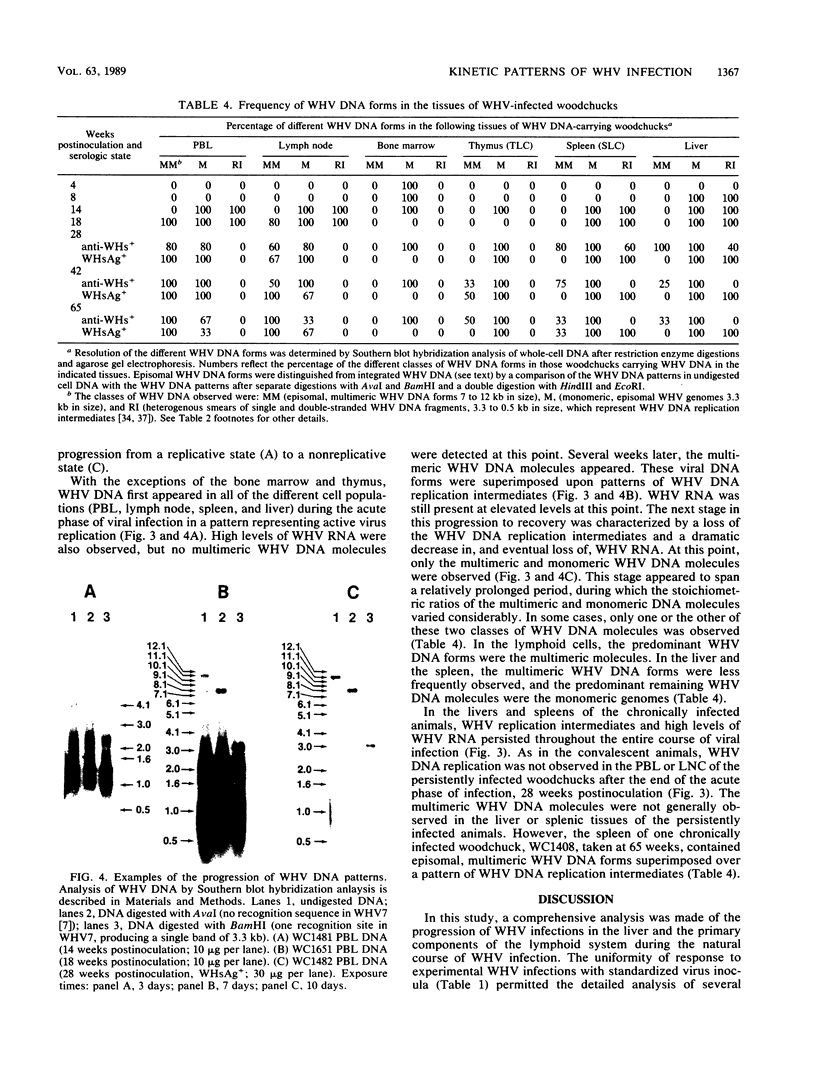
In this study, the kinetic patterns of woodchuck hepatitis virus (WHV) infection were monitored in the liver and the five primary components of the lymphoid system (peripheral blood lymphocytes, lymph nodes, bone marrow, spleen, and thymus). Groups of woodchucks experimentally infected with a standardized inoculum of WHV were sacrificed at different times over a 65-week period beginning in the preacute phase of viral infection and continuing to the period of serologic recovery or the establishment of chronic infections and subsequent hepatocellular carcinoma. Infection by WHV was not limited to the liver but involved the major components of the lymphoid system during all stages of virus infection. A complex series of kinetic patterns was observed for the appearance of WHV DNA in the different lymphoid compartments and the liver during the entire course of viral infection. A progressive evolution of different WHV genomic forms related to the replicative state of WHV was also observed. Lymphoid cells of the bone marrow were the first cells in which WHV DNA was detected, followed in order by the liver, the spleen, peripheral blood lymphocytes, lymph nodes, and finally the thymus. Several differences were observed in the cellular WHV DNA patterns between woodchucks that developed chronic WHV infections and those that serologically recovered from acute WHV infections. The observations compiled in this study indicate that the host lymphoid system is intimately involved in the natural history of hepadnavirus infections from the earliest stages of virus entry.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G. J., Mondelli M., Naumov N. V., Nouriaria K. T., Vergani D., Lowe D., Eddleston A. L., Williams R. Functional characterization of peripheral blood lymphocytes in chronic HBsAg carriers. Clin Exp Immunol. 1986 Mar;63(3):498–507. [PMC free article] [PubMed] [Google Scholar]
- Babinet C., Farza H., Morello D., Hadchouel M., Pourcel C. Specific expression of hepatitis B surface antigen (HBsAg) in transgenic mice. Science. 1985 Dec 6;230(4730):1160–1163. doi: 10.1126/science.3865370. [DOI] [PubMed] [Google Scholar]
- Burk R. D., DeLoia J. A., elAwady M. K., Gearhart J. D. Tissue preferential expression of the hepatitis B virus (HBV) surface antigen gene in two lines of HBV transgenic mice. J Virol. 1988 Feb;62(2):649–654. doi: 10.1128/jvi.62.2.649-654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis E., Abraham K. G., Miller R. W. Modulation of the immunological response to hepatitis B virus by antibodies. Hepatology. 1987 May-Jun;7(3):563–568. doi: 10.1002/hep.1840070324. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Castle K. L., Xavier C., Anderson D. S. Functional properties of lymphocyte subpopulations in hepatitis B virus infection. I. Suppressor cell control of T lymphocyte responsiveness. J Immunol. 1981 Jan;126(1):38–44. [PubMed] [Google Scholar]
- Cohen J. I., Miller R. H., Rosenblum B., Denniston K., Gerin J. L., Purcell R. H. Sequence comparison of woodchuck hepatitis virus replicative forms shows conservation of the genome. Virology. 1988 Jan;162(1):12–20. doi: 10.1016/0042-6822(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Davis G. L., Hoofnagle J. H., Waggoner J. G. Spontaneous reactivation of chronic hepatitis B virus infection. Gastroenterology. 1984 Feb;86(2):230–235. [PubMed] [Google Scholar]
- Davison F., Alexander G. J., Anastassakos C., Fagan E. A., Williams R. Leucocyte hepatitis B virus DNA in acute and chronic hepatitis B virus infection. J Med Virol. 1987 Aug;22(4):379–385. doi: 10.1002/jmv.1890220411. [DOI] [PubMed] [Google Scholar]
- Davison F., Alexander G. J., Trowbridge R., Fagan E. A., Williams R. Detection of hepatitis B virus DNA in spermatozoa, urine, saliva and leucocytes, of chronic HBsAg carriers. A lack of relationship with serum markers of replication. J Hepatol. 1987 Feb;4(1):37–44. doi: 10.1016/s0168-8278(87)80007-7. [DOI] [PubMed] [Google Scholar]
- Freiman J. S., Jilbert A. R., Dixon R. J., Holmes M., Gowans E. J., Burrell C. J., Wills E. J., Cossart Y. E. Experimental duck hepatitis B virus infection: pathology and evolution of hepatic and extrahepatic infection. Hepatology. 1988 May-Jun;8(3):507–513. doi: 10.1002/hep.1840080313. [DOI] [PubMed] [Google Scholar]
- Fukuda R., Fukumoto S., Shimada Y. A sequential study of viral DNA in serum in experimental transmission of duck hepatitis B virus. J Med Virol. 1987 Apr;21(4):311–320. doi: 10.1002/jmv.1890210403. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., England J. M., Deery D. T., Petcu D. J., Mason W. S., Molnar-Kimber K. L. Viral nucleic acid synthesis and antigen accumulation in pancreas and kidney of Pekin ducks infected with duck hepatitis B virus. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4865–4869. doi: 10.1073/pnas.80.15.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoar D. I., Bowen T., Matheson D., Poon M. C. Hepatitis B virus DNA is enriched in polymorphonuclear leukocytes. Blood. 1985 Dec;66(6):1251–1253. [PubMed] [Google Scholar]
- Jameel S., Siddiqui A. The human hepatitis B virus enhancer requires trans-acting cellular factor(s) for activity. Mol Cell Biol. 1986 Feb;6(2):710–715. doi: 10.1128/mcb.6.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilbert A. R., Freiman J. S., Gowans E. J., Holmes M., Cossart Y. E., Burrell C. J. Duck hepatitis B virus DNA in liver, spleen, and pancreas: analysis by in situ and Southern blot hybridization. Virology. 1987 Jun;158(2):330–338. doi: 10.1016/0042-6822(87)90205-4. [DOI] [PubMed] [Google Scholar]
- Korba B. E., Cote P. J., Gerin J. L. Mitogen-induced replication of woodchuck hepatitis virus in cultured peripheral blood lymphocytes. Science. 1988 Sep 2;241(4870):1213–1216. doi: 10.1126/science.3261887. [DOI] [PubMed] [Google Scholar]
- Korba B. E., Gowans E. J., Wells F. V., Tennant B. C., Clarke R., Gerin J. L. Systemic distribution of woodchuck hepatitis virus in the tissues of experimentally infected woodchucks. Virology. 1988 Jul;165(1):172–181. doi: 10.1016/0042-6822(88)90670-8. [DOI] [PubMed] [Google Scholar]
- Korba B. E., Wells F., Tennant B. C., Cote P. J., Gerin J. L. Lymphoid cells in the spleens of woodchuck hepatitis virus-infected woodchucks are a site of active viral replication. J Virol. 1987 May;61(5):1318–1324. doi: 10.1128/jvi.61.5.1318-1324.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Wells F., Tennant B. C., Yoakum G. H., Purcell R. H., Gerin J. L. Hepadnavirus infection of peripheral blood lymphocytes in vivo: woodchuck and chimpanzee models of viral hepatitis. J Virol. 1986 Apr;58(1):1–8. doi: 10.1128/jvi.58.1.1-8.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Wilson V. L., Yoakum G. H. Induction of hepatitis B virus core gene in human cells by cytosine demethylation in the promoter. Science. 1985 May 31;228(4703):1103–1106. doi: 10.1126/science.2581318. [DOI] [PubMed] [Google Scholar]
- Lieberman H. M., Tung W. W., Shafritz D. A. Splenic replication of hepatitis B virus in the chimpanzee chronic carrier. J Med Virol. 1987 Apr;21(4):347–359. doi: 10.1002/jmv.1890210407. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Robinson W. S. Integrated hepatitis B virus DNA sequences specifying the major viral core polypeptide are methylated in PLC/PRF/5 cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2534–2538. doi: 10.1073/pnas.80.9.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möröy T., Etiemble J., Trépo C., Tiollais P., Buendia M. A. Transcription of woodchuck hepatitis virus in the chronically infected liver. EMBO J. 1985 Jun;4(6):1507–1514. doi: 10.1002/j.1460-2075.1985.tb03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Kamiyama I., Inomata M., Imai M., Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med. 1976 Apr 1;294(14):746–749. doi: 10.1056/NEJM197604012941402. [DOI] [PubMed] [Google Scholar]
- Pasquinelli C., Lauré F., Chatenoud L., Beaurin G., Gazengel C., Bismuth H., Degos F., Tiollais P., Bach J. F., Bréchot C. Hepatitis B virus DNA in mononuclear blood cells. A frequent event in hepatitis B surface antigen-positive and -negative patients with acute and chronic liver disease. J Hepatol. 1986;3(1):95–103. doi: 10.1016/s0168-8278(86)80152-0. [DOI] [PubMed] [Google Scholar]
- Perrillo R. P., Campbell C. R., Sanders G. E., Regenstein F. G., Bodicky C. J. Spontaneous clearance and reactivation of hepatitis B virus infection among male homosexuals with chronic type B hepatitis. Ann Intern Med. 1984 Jan;100(1):43–46. doi: 10.7326/0003-4819-100-1-43. [DOI] [PubMed] [Google Scholar]
- Ponzetto A., Cote P. J., Ford E. C., Purcell R. H., Gerin J. L. Core antigen and antibody in woodchucks after infection with woodchuck hepatitis virus. J Virol. 1984 Oct;52(1):70–76. doi: 10.1128/jvi.52.1.70-76.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper H., Roth L., Purcell R. H., Tennant B. C., Gerin J. L. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1987 Feb;84(3):866–870. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romet-Lemonne J. L., McLane M. F., Elfassi E., Haseltine W. A., Azocar J., Essex M. Hepatitis B virus infection in cultured human lymphoblastoid cells. Science. 1983 Aug 12;221(4611):667–669. doi: 10.1126/science.6867736. [DOI] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science. 1986 Apr 25;232(4749):477–484. doi: 10.1126/science.3961490. [DOI] [PubMed] [Google Scholar]
- Shen H. D., Choo K. B., Wu T. C., Ng H. T., Han S. H. Hepatitis B virus infection of cord blood leukocytes. J Med Virol. 1987 Jul;22(3):211–216. doi: 10.1002/jmv.1890220303. [DOI] [PubMed] [Google Scholar]
- Strober S. Natural suppressor (NS) cells, neonatal tolerance, and total lymphoid irradiation: exploring obscure relationships. Annu Rev Immunol. 1984;2:219–237. doi: 10.1146/annurev.iy.02.040184.001251. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Tagawa M., Omata M., Yokosuka O., Uchiumi K., Imazeki F., Okuda K. Early events in duck hepatitis B virus infection. Sequential appearance of viral deoxyribonucleic acid in the liver, pancreas, kidney, and spleen. Gastroenterology. 1985 Dec;89(6):1224–1229. [PubMed] [Google Scholar]
- Tagawa M., Robinson W. S., Marion P. L. Duck hepatitis B virus replicates in the yolk sac of developing embryos. J Virol. 1987 Jul;61(7):2273–2279. doi: 10.1128/jvi.61.7.2273-2279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tong M. J., Sampliner R. E., Govindarajan S., Co R. L. Spontaneous reactivation of hepatitis B in Chinese patients with HBsAg-positive chronic active hepatitis. Hepatology. 1987 Jul-Aug;7(4):713–718. doi: 10.1002/hep.1840070416. [DOI] [PubMed] [Google Scholar]
- Vento S., Rondanelli E. G., Ranieri S., O'Brien C. J., Williams R., Eddleston A. L. Prospective study of cellular immunity to hepatitis-B-virus antigens from the early incubation phase of acute hepatitis B. Lancet. 1987 Jul 18;2(8551):119–122. doi: 10.1016/s0140-6736(87)92329-4. [DOI] [PubMed] [Google Scholar]