Abstract
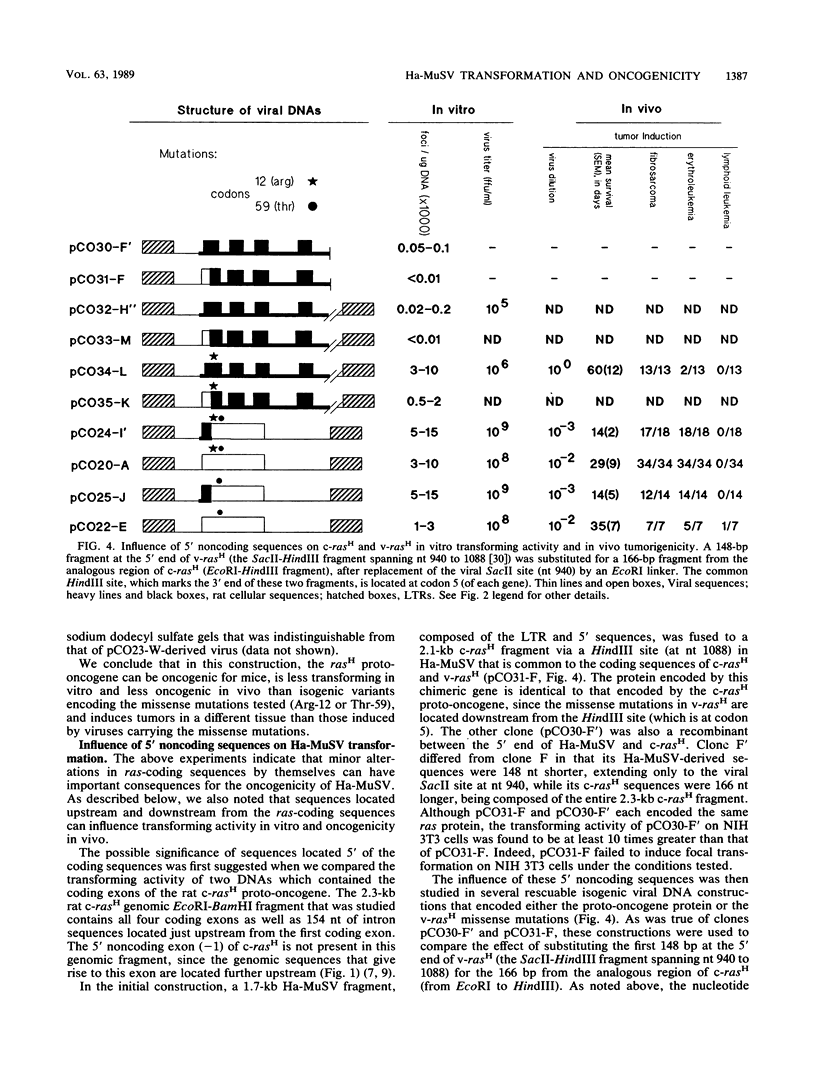
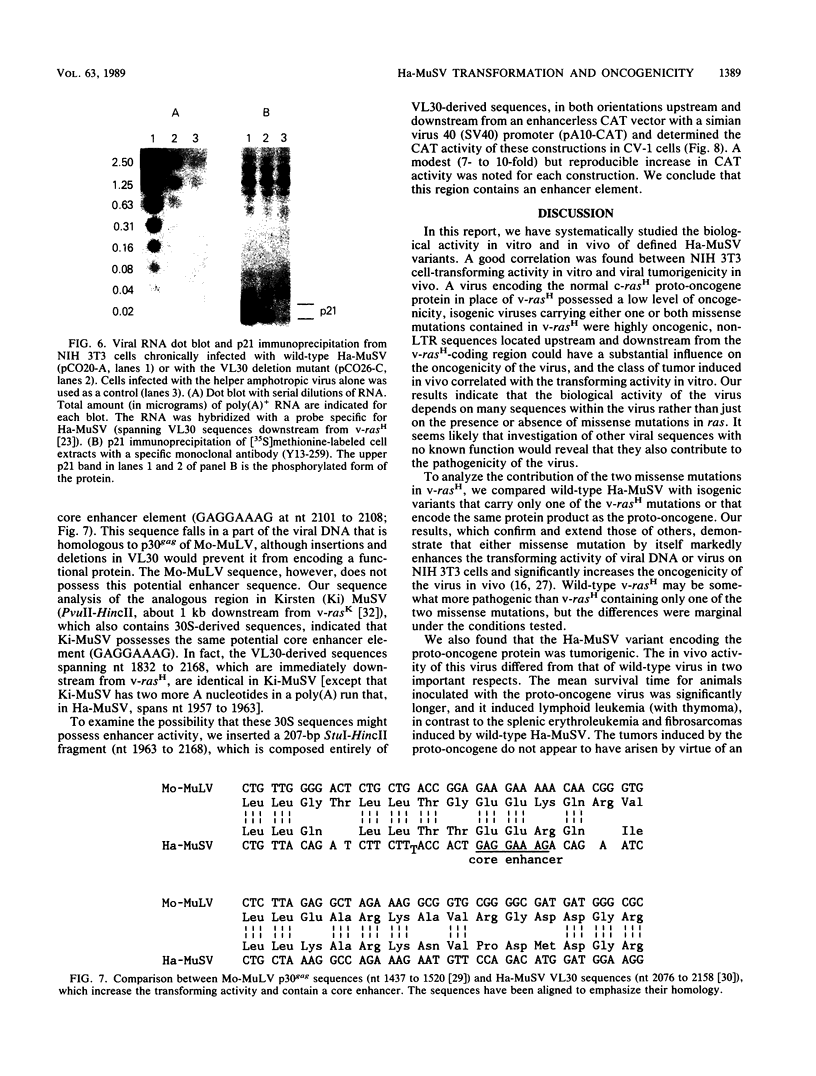
The rat-derived Harvey murine sarcoma virus (Ha-MuSV) contains a transduced ras oncogene activated by two missense mutations and flanked by rat retroviruslike VL30 sequences. Ha-MuSV induces focal transformation of mouse NIH 3T3 cells in vitro and tumors (fibrosarcomas and splenic erythroleukemias) in newborn mice. We have used these two assays to study the contribution of coding and noncoding viral sequences to the biological activity of Ha-MuSV. A good correlation was found between the in vitro and in vivo assays. In several different isogenic Ha-MuSV variants, those with a rasH gene that had one or both of the Ha-MuSV missense mutations were much more active biologically than the corresponding proto-oncogene. A Ha-MuSV variant that encoded the proto-oncogene protein induced lymphoid leukemias (with thymomas), with a relatively long latent period, rather than the fibrosarcomas and erythroleukemias characteristic of Ha-MuSV with one or both missense mutations. A VL30-derived segment with enhancer activity was identified downstream from v-rasH. A mutant Ha-MuSV from which this 3' noncoding segment was deleted expressed lower levels of the wild-type viral protein, displayed impaired transforming activity in vitro, and induced lymphoid leukemias (with thymomas). 5' noncoding rat c-rasH sequences were found to increase the biological activity of the virus when substituted for the corresponding segment of v-rasH. We conclude that (i) the biological activity of Ha-MuSV can be influence significantly by noncoding sequences located outside the long terminal repeat as well as by coding sequences, (ii) VL30 sequences positively regulate the expression of v-rasH, (iii) relatively low biological levels of ras, whether resulting from low-level expression of wild type v-rasH or high-levels of ras proto-oncogene protein, induce a type of tumor that differs from tumors induced by high biological levels of ras, and (iv) the in vivo pathogenicity of the Ha-MuSV variants correlated with their transforming activity on NIH 3T3 cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Berns A. J., Lai M. H., Bosselman R. A., McKennett M. A., Bacheler L. T., Fan H., Maandag E. C., van der Putten H. V., Verma I. M. Molecular cloning of unintegrated and a portion of integrated moloney murine leukemia viral DNA in bacteriophage lambda. J Virol. 1980 Oct;36(1):254–263. doi: 10.1128/jvi.36.1.254-263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg K., Ryden T. A., Beemon K. Localization and footprinting of an enhancer within the avian sarcoma virus gag gene. J Virol. 1988 May;62(5):1617–1624. doi: 10.1128/jvi.62.5.1617-1624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Ellis R. W., Scolnick E. M., Lowy D. R. Transformation by cloned Harvey murine sarcoma virus DNA: efficiency increased by long terminal repeat DNA. Science. 1980 Dec 12;210(4475):1249–1251. doi: 10.1126/science.6254153. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Morgan P. L., Lee E. J., Pirollo K. F., White E. A., Patrick D. H., Tsichlis P. N. Pathogenicity of retroviruses containing either the normal human c-Ha-ras1 gene or its mutated form derived from the bladder carcinoma EJ/T24 cell line. J Exp Pathol. 1985 Fall;2(3):177–189. [PubMed] [Google Scholar]
- Chattopadhyay S. K., Oliff A. I., Linemeyer D. L., Lander M. R., Lowy D. R. Genomes of murine leukemia viruses isolated from wild mice. J Virol. 1981 Sep;39(3):777–791. doi: 10.1128/jvi.39.3.777-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichutek K., Duesberg P. H. Harvey ras genes transform without mutant codons, apparently activated by truncation of a 5' exon (exon -1). Proc Natl Acad Sci U S A. 1986 Apr;83(8):2340–2344. doi: 10.1073/pnas.83.8.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damante G., Filetti S., Rapoport B. Nucleotide sequence and characterization of the 5' flanking region of the rat Ha-ras protooncogene. Proc Natl Acad Sci U S A. 1987 Feb;84(3):774–778. doi: 10.1073/pnas.84.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Cancer genes: rare recombinants instead of activated oncogenes (a review). Proc Natl Acad Sci U S A. 1987 Apr;84(8):2117–2124. doi: 10.1073/pnas.84.8.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Maryak J. M., Young H. A., Shih T. Y., Chang E. H., Lowy D. R., Scolnick E. M. Dual evolutionary origin for the rat genetic sequences of Harvey murine sarcoma virus. J Virol. 1980 Nov;36(2):408–420. doi: 10.1128/jvi.36.2.408-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY J. J. AN UNIDENTIFIED VIRUS WHICH CAUSES THE RAPID PRODUCTION OF TUMOURS IN MICE. Nature. 1964 Dec 12;204:1104–1105. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P., Androphy E. J., Lowy D. R., Schiller J. T. The specific DNA recognition sequence of the bovine papillomavirus E2 protein is an E2-dependent enhancer. EMBO J. 1988 Feb;7(2):525–531. doi: 10.1002/j.1460-2075.1988.tb02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Santos E., Notario V., Barbacid M., Yamazaki S., Kung H., Seamans C., McAndrew S., Crowl R. Expression of normal and transforming H-ras genes in Escherichia coli and purification of their encoded p21 proteins. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5305–5309. doi: 10.1073/pnas.81.17.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Srivastava S. K., Anderson P. S., Aaronson S. A. Ras p21 proteins with high or low GTPase activity can efficiently transform NIH/3T3 cells. Cell. 1986 Feb 28;44(4):609–617. doi: 10.1016/0092-8674(86)90270-9. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Golemis E., Hartley J. W., Hopkins N. Disease specificity of nondefective Friend and Moloney murine leukemia viruses is controlled by a small number of nucleotides. J Virol. 1987 Mar;61(3):693–700. doi: 10.1128/jvi.61.3.693-700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield J. W. NIH 3T3 cell line. Science. 1982 Oct 15;218(4569):214–216. doi: 10.1126/science.218.4569.214-b. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Willumsen B. M. The ras gene family. Cancer Surv. 1986;5(2):275–289. [PubMed] [Google Scholar]
- Reddy E. P., Lipman D., Andersen P. R., Tronick S. R., Aaronson S. A. Nucleotide sequence analysis of the BALB/c murine sarcoma virus transforming gene. J Virol. 1985 Mar;53(3):984–987. doi: 10.1128/jvi.53.3.984-987.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop D. R., Lowy D. R., Tambourin P. E., Strickland J., Harper J. R., Balaschak M., Spangler E. F., Yuspa S. H. An activated Harvey ras oncogene produces benign tumours on mouse epidermal tissue. 1986 Oct 30-Nov 5Nature. 323(6091):822–824. doi: 10.1038/323822a0. [DOI] [PubMed] [Google Scholar]
- Ruta M., Wolford R., Dhar R., Defeo-Jones D., Ellis R. W., Scolnick E. M. Nucleotide sequence of the two rat cellular rasH genes. Mol Cell Biol. 1986 May;6(5):1706–1710. doi: 10.1128/mcb.6.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R. D., Oliff A. The pathophysiology of murine retrovirus-induced leukemias. Crit Rev Oncol Hematol. 1986;5(3):257–323. doi: 10.1016/s1040-8428(86)80041-5. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Colby W. W., Capon D. J., Goeddel D. V., Levinson A. D. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature. 1984 Nov 1;312(5989):71–75. doi: 10.1038/312071a0. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O. Oncogenes and cancer: the p21 ras genes. Cancer Invest. 1984;2(2):109–123. doi: 10.3109/07357908409020294. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Ryder T., Ohtsubo E. Nucleotide sequence of the oncogene encoding the p21 transforming protein of Kirsten murine sarcoma virus. Science. 1982 Sep 3;217(4563):937–939. doi: 10.1126/science.6287573. [DOI] [PubMed] [Google Scholar]
- Velu T. J., Beguinot L., Vass W. C., Willingham M. C., Merlino G. T., Pastan I., Lowy D. R. Epidermal-growth-factor-dependent transformation by a human EGF receptor proto-oncogene. Science. 1987 Dec 4;238(4832):1408–1410. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Christensen A., Hubbert N. L., Papageorge A. G., Lowy D. R. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984 Aug 16;310(5978):583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Papageorge A. G., Hubbert N., Bekesi E., Kung H. F., Lowy D. R. Transforming p21 ras protein: flexibility in the major variable region linking the catalytic and membrane-anchoring domains. EMBO J. 1985 Nov;4(11):2893–2896. doi: 10.1002/j.1460-2075.1985.tb04019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen B. M., Papageorge A. G., Hubbert N., Bekesi E., Kung H. F., Lowy D. R. Transforming p21 ras protein: flexibility in the major variable region linking the catalytic and membrane-anchoring domains. EMBO J. 1985 Nov;4(11):2893–2896. doi: 10.1002/j.1460-2075.1985.tb04019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]