Abstract
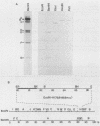
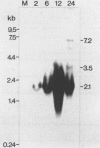
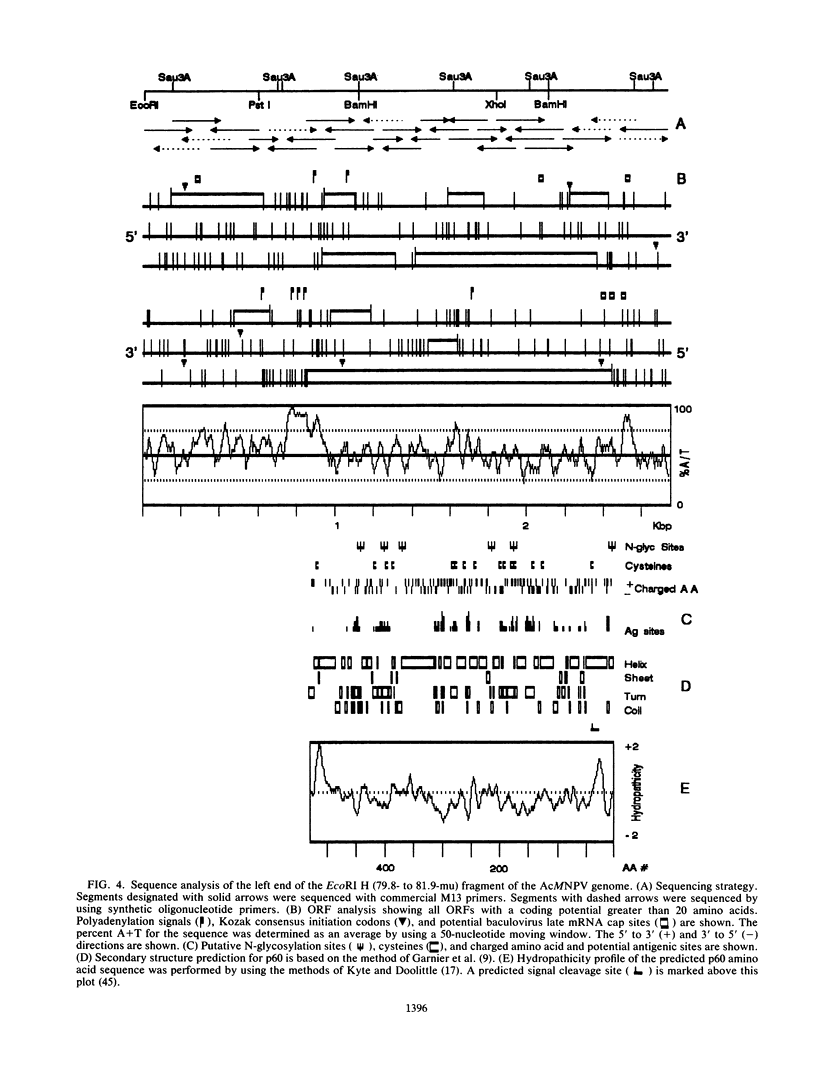
Monoclonal antibodies with specificity for the abundant envelope surface glycoprotein (gp67) of Autographa californica nuclear polyhedrosis virus (AcMNPV) were used to screen a lambda gt11 expression library of AcMNPV DNA fragments. The gp67 gene was mapped to the left end of the EcoRI H fragment in a right-to-left orientation on the consensus map of AcMNPV. A 2.1-kilobase transcript which hybridized to the region was first detected in cell extracts at 2 h postinfection; it peaked in abundance at 18 h postinfection and thereafter was present at lower levels. The nucleotide sequence of the region was determined, and a 1,590-nucleotide open reading frame flanked by an AT-rich sequence was identified that could encode a polypeptide with 529 amino acid residues (molecular mass of 60,167 daltons). Computer analysis indicated that the peptide possesses two hydrophobic regions near the N and C termini as well as six potential N-linked glycosylation sites. We suggest that following cleavage of a signal peptide, the polypeptide undergoes further processing and becomes anchored at its C terminus in the virus envelope. The final seven amino acid residues at the C terminus contain basic amino acids and may have a role in virion assembly.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blinov V. M., Gutorov V. V., Holodilov N. G., Iljichev A. A., Karginov V. A., Mikrjukov N. N., Mordvinov V. A., Nikonov I. V., Petrov N. A., Urmanov I. H. Nucleotide sequence of the Galleria mellonella nuclear polyhedrosis virus origin of DNA replication. FEBS Lett. 1984 Feb 27;167(2):254–258. doi: 10.1016/0014-5793(84)80137-4. [DOI] [PubMed] [Google Scholar]
- Brown M., Crawford A. M., Faulkner P. Genetic Analysis of a Baculovirus, Autographa californica Nuclear Polyhedrosis Virus I. Isolation of Temperature-Sensitive Mutants and Assortment into Complementation Groups. J Virol. 1979 Jul;31(1):190–198. doi: 10.1128/jvi.31.1.190-198.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Carstens E. B., Eaton B. T., Faulkner P. Molecular Cloning and Physical Mapping of Restriction Endonuclease Fragments of Autographa californica Nuclear Polyhedrosis Virus DNA. J Virol. 1982 Mar;41(3):940–946. doi: 10.1128/jvi.41.3.940-946.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Friesen P. D., Miller L. K. Temporal regulation of baculovirus RNA: overlapping early and late transcripts. J Virol. 1985 May;54(2):392–400. doi: 10.1128/jvi.54.2.392-400.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Goldstein N. I., McIntosh A. H. Glycoproteins of nuclear polyhedrosis viruses. Arch Virol. 1980;64(2):119–126. doi: 10.1007/BF01318015. [DOI] [PubMed] [Google Scholar]
- Hohmann A. W., Faulkner P. Monoclonal antibodies to baculovirus structural proteins: determination of specificities by Western blot analysis. Virology. 1983 Mar;125(2):432–444. doi: 10.1016/0042-6822(83)90214-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lübbert H., Doerfler W. Transcription of overlapping sets of RNAs from the genome of Autographa californica nuclear polyhedrosis virus: a novel method for mapping RNAs. J Virol. 1984 Oct;52(1):255–265. doi: 10.1128/jvi.52.1.255-265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainprize T. H., Lee K., Miller L. K. Variation in the temporal expression of overlapping baculovirus transcripts. Virus Res. 1986 Oct;6(1):85–99. doi: 10.1016/0168-1702(86)90059-6. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Haarr L., Preston C. M. Processing of herpes simplex virus proteins and evidence that translation of thymidine kinase mRNA is initiated at three separate AUG codons. J Virol. 1983 May;46(2):434–445. doi: 10.1128/jvi.46.2.434-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oellig C., Happ B., Müller T., Doerfler W. Overlapping sets of viral RNAs reflect the array of polypeptides in the EcoRI J and N fragments (map positions 81.2 to 85.0) of the Autographa californica nuclear polyhedrosis virus genome. J Virol. 1987 Oct;61(10):3048–3057. doi: 10.1128/jvi.61.10.3048-3057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Rohrmann G. F. Polyhedrin structure. J Gen Virol. 1986 Aug;67(Pt 8):1499–1513. doi: 10.1099/0022-1317-67-8-1499. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Adams G. A., Gallione C. J. The presence of cysteine in the cytoplasmic domain of the vesicular stomatitis virus glycoprotein is required for palmitate addition. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2050–2054. doi: 10.1073/pnas.81.7.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjia S. T., Carstens E. B., Doerfler W. Infection of Spodoptera frugiperda cells with Autographa californica nuclear polyhedrosis virus II. The viral DNA and the kinetics of its replication. Virology. 1979 Dec;99(2):399–409. doi: 10.1016/0042-6822(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Vaughn J. L., Goodwin R. H., Tompkins G. J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro. 1977 Apr;13(4):213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- Vlak J. M., Smith G. E. Orientation of the Genome of Autographa californica Nuclear Polyhedrosis Virus: a Proposal. J Virol. 1982 Mar;41(3):1118–1121. doi: 10.1128/jvi.41.3.1118-1121.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman L. E., Summers M. D. Autographa californica nuclear polyhedrosis virus: comparative infectivity of the occluded, alkali-liberated, and nonoccluded forms. J Invertebr Pathol. 1977 Jul;30(1):102–103. doi: 10.1016/0022-2011(77)90045-3. [DOI] [PubMed] [Google Scholar]
- Volkman L. E., Summers M. D., Hsieh C. H. Occluded and nonoccluded nuclear polyhedrosis virus grown in Trichoplusia ni: comparative neutralization comparative infectivity, and in vitro growth studies. J Virol. 1976 Sep;19(3):820–832. doi: 10.1128/jvi.19.3.820-832.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman L. E. The 64K envelope protein of budded Autographa californica nuclear polyhedrosis virus. Curr Top Microbiol Immunol. 1986;131:103–118. doi: 10.1007/978-3-642-71589-1_6. [DOI] [PubMed] [Google Scholar]
- Weyer U., Possee R. D. Functional analysis of the p10 gene 5' leader sequence of the Autographa californica nuclear polyhedrosis virus. Nucleic Acids Res. 1988 May 11;16(9):3635–3653. doi: 10.1093/nar/16.9.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]