Abstract
Numerous studies have shown that the levels of matrix metalloproteinase (MMP)-2 and/or MMP-9 are associated with the invasive phenotypes of cancer cells. This study investigated the effects of caffeic acid phenethyl ester (CAPE), a chemopreventive phytochemical derived from honeybee propolis, on the invasive phenotype of SK-Hep1 human hepatocellular carcinoma cells (SK-Hep1 cells). CAPE effectively suppressed SK-Hep1 cell invasion in a dose-dependent manner. The constitutive expression of MMP-2 and MMP-9 in SK-Hep1 cells was almost completely abolished by treatment with 12.5 μM CAPE. CAPE also significantly inhibited nuclear factor kappa B (NF-κB) DNA-binding activity in SK-Hep1 cells. These results taken together suggest that CAPE exerts antimetastatic potential through inhibition of MMP-2 and MMP-9 expression, possibly by targeting NF-κB in hepatocellular carcinoma.
Keywords: Caffeic acid phenethyl ester, Hepatocellular carcinoma, Invasion, Matrix metalloproteinases, Nuclear factor kappa B
Introduction
Metastasis is one of the major causes of mortality in cancer patients and occurs as a complex multistep process that involves cancer cell adhesion, invasion, and migration [5]. Tumor cell invasion and metastasis are complex mechanisms involving extracellular matrix (ECM)-degrading proteinase activity and migration through the ECM. Matrix metalloproteinases (MMPs) are enzymes involved in ECM degradation and are linked to various steps in metastasis development [1]. Among MMPs, the type IV collagenases, such as MMP-2 and MMP-9, have been most strongly linked to tumor-cell invasion [13]. One of the prime components of the intracellular signaling pathways responsible for MMP-2 and MMP-9 induction is the eukaryotic transcription factor nuclear factor kappa B (NF-κB) [6].
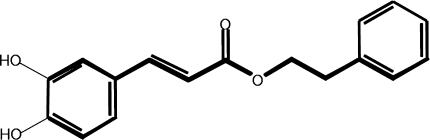
Recently, much attention has been devoted to identifying chemopreventive phytochemicals derived from the daily diet. Caffeic acid phenethyl ester (CAPE, Fig. 1) is an active component of the propolis obtained from honeybee hives that exhibits antioxidant, anti-inflammatory, anticarcinogenic, and immunomodulatory activities in diverse systems. CAPE induces apoptosis and inhibits expression of the transformed phenotype in transformed cells [12]. CAPE also inhibits the azoxymethane-induced formation of aberrant crypts that are relevant to colon carcinogenesis [2]. We previously reported that CAPE inhibited cyclooxygenase-2 (COX-2) expression and restored gap-junction intercellular communication in H-Ras-transformed rat liver epithelial cells [7].
Fig. 1.
The chemical structure of caffeic acid phenethyl ester (CAPE)
Hepatocellular carcinoma is one of the most lethal malignancies and ranks as the second leading cause of cancer deaths in Southeast Asia [4]. The SK-Hep1 human hepatocellular carcinoma cell (SK-Hep1 cell) line is invasive and expresses the gelatinase activity required for invasion [8]. To investigate the chemopreventive effect of CAPE on hepatocarcinogenesis, we assessed whether CAPE inhibits the invasive phenotype of SK-Hep1 cells. Here we report that CAPE appears to effectively inhibit the invasive phenotype through downregulation of MMP-2 and MMP-9 by blocking NF-κB activation.
Materials and methods
Chemicals
CAPE and mitomycin C were obtained from Sigma Chemical (St Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM); fetal bovine serum; penicillin-streptomycin; M199; heparin, an NF-κB DNA-binding detection system, and Trizol® were purchased from Gibco BRL (Grand Island, NY, USA). All other chemicals used were of analytical grade (Fisher, Springfield, NJ, USA).
Cell lines and culture conditions
SK-Hep1 cells were purchased from the Korean Cell Line Bank (Seoul, South Korea). The cells were cultured in DMEM supplemented with 10% fetal bovine serum and penicillin-streptomycin. The cells were maintained in a humidified atmosphere with 95% air and 5% CO2 at 37°C.
In vitro invasion assay
An in vitro invasion assay was performed using a 24-well transwell unit with polycarbonate filters (Corning Costar, Cambridge, MA, USA) as described previously [5]. The lower side of the filter was coated with type I collagen, and the upper side was coated with Matrigel (Collaborative Research, Lexington, KY, USA). The lower compartment was filled with serum-free media containing 0.1% bovine serum albumin (BSA). The SK-Hep1 cells were suspended in 100 μl of serum-free media containing CAPE at various concentrations and placed in the upper part of the transwell plate, incubated for 24 h, fixed with methanol, and stained with hematoxylin for 10 min followed briefly by eosin. The invasive phenotype was assessed by counting the cells that migrated to the lower side of the filter under light microscopy at ×400 magnification. Thirteen fields were counted for each filter, and each sample was assayed in triplicate.
Gelatin zymography assay
SK-Hep1 cells cultured in serum-free medium were treated with CAPE at various concentrations for 48 h. Conditioned medium was collected and centrifuged to remove cell debris. A gelatin zymography assay was performed as described previously [5]. Briefly, equal amounts of conditioned media were mixed with 2× Laemmli nonreducing sample buffer, incubated for 15 min at room temperature, and electrophoresed on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels containing 1 mg/ml gelatin. After electrophoresis, the gels were washed and incubated overnight at 37°C. After staining with 0.1% Coomassie brilliant blue, areas of lysis were observed as white bands against a blue background.
Electrophoretic mobility shift assay (EMSA)
An electrophoretic mobility shift assay (EMSA) was performed using a DNA-binding detection kit (GIBCO BRL, Grand Island, NY, USA) according to the manufacturer’s instructions. Briefly, the NF-κB oligonucleotide probe (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) was labeled with [γ-32P]adenosine triphosphate (ATP) by T4 polynucleotide kinase and purified on a Nick column (Amersham Pharmacia Biotech, Buckinghamshire, UK). The binding reaction was carried out in 25 μl of the mixture containing 10 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM DTT, 1 mM EDTA, 4% (v/v) glycerol, 0.1 mg/ml sonicated salmon sperm DNA, 10 μg of nuclear extracts, and 100,000 cpm of the labeled probe. After 50 min incubation at room temperature, 2 μl of 0.1% bromophenol blue was added, and samples were electrophoresed through a 6% nondenaturing polyacrylamide gel at 150 V in a cold room for 2 h. Finally, the gel was dried and exposed to X-ray film.
Results
CAPE inhibits the invasive phenotype of SK-Hep1 cells
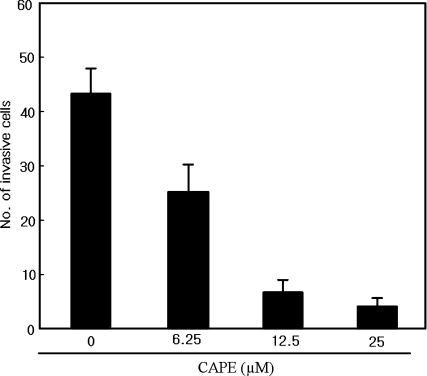
An in vitro invasion assay was applied to highly invasive SK-Hep1 cells to investigate the antimetastatic potential of CAPE. Treatment with CAPE for 24 h dose-dependently inhibited the invasive phenotype of SK-Hep1 cells (Fig. 2), with CAPE at 12.5 μM reducing the number of cells that invaded through a reconstituted basement membrane by 85%. The CAPE treatment did not affect cell viability (data not shown), indicating that this inhibitory effect of CAPE was not due to cytotoxicity.
Fig. 2.
Inhibition of the invasive phenotype of SK-Hep1 human hepatocellular carcinoma cells (SK-Hep1 cells). Treated with caffeic acid phenethyl ester (CAPE) in vitro, invasion assay was applied to cells (2 × 104) treated with CAPE at various concentrations for 24 h. The number of cells that invaded per field was counted under light microscopy (×400 magnification). The mean and standard error of mean values of triplicate experiments are shown
CAPE inhibits expression of MMP-2 and MMP-9 in SK-Hep1 cells
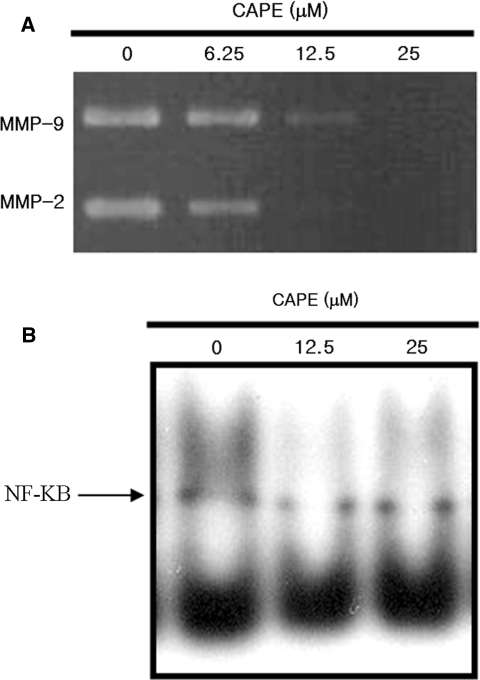
The invasive phenotype of cancer cells is strongly associated with increased levels of MMP-2 and/or MMP-9. To determine the effect of CAPE on MMP-2 and/or MMP-9 expression, SK-Hep1 cells were treated with CAPE at various concentrations for 48 h in serum-free medium. The results from a gelatin-based zymography assay demonstrated that CAPE suppressed both MMP-2 and MMP-9 expression in a dose-dependent manner (Fig. 3). These results suggest that the inhibitory effect of CAPE on invasion of SK-Hep1 is closely related to downregulation of both MMP-2 and MMP-9.
Fig. 3.
Effects of caffeic acid phenethyl ester (CAPE) on expression of matrix metalloproteinase (MMP)-2, MMP-3, and nuclear factor kappa B (NF-κB) DNA-binding activity in SK-Hep1 cells. a CAPE inhibits MMP-2 and MMP-9 expression in SK-Hep1 cells. Cells were treated with CAPE for 48 h. The activities of secreted MMP-2 (72 kDa) and MMP-9 (92 kDa) in the conditioned media were determined by gelatin zymography. b CAPE inhibits NF-κB DNA-binding activity in SK-Hep1 cells. SK-Hep1 cells were treated with CAPE for 1 h. The DNA-binding activity of NF-κB activation was determined by an electrophoretic mobility shift assay as described in “Materials and methods”, where CAPE was used at 12.5 and 25 μM. Data are representative of two independent experiments
CAPE inhibits NF-κB activation in SK-Hep1 cells
There are multiple lines of evidence that NF-κB plays a key role in the expression of MMP-2 and MMP-9 [3, 10, 11]. Although the molecular basis of the cancer chemopreventive activity of CAPE remains to be clarified, it was suggested that CAPE is a potent and specific inhibitor of NF-κB [9]. We conducted an EMSA to examine whether CAPE inhibits NF-κB DNA-binding activity. The DNA-binding activity of NF-κB was prominently inhibited in SK-Hep1 cells treated with CAPE at 12.5 and 25 μM for 24 h (Fig. 3b). These results reveal that NF-κB is a potential molecular target for inhibition of MMP-2 and MMP-9 in SK-Hep1 cells by CAPE.
Discussion
Hepatocellular carcinoma is among the most prevalent and deadly cancers worldwide, and identifying and developing pharmaceutical agents that can inhibit the processes of invasion and metastasis can provide an feasible strategy for its prevention and treatment. CAPE has been shown to inhibit COX-2 expression and restore gap-junction intercellular communication of H-Ras-transformed rat liver epithelial cells [7]. The major aim of this investigation was to elucidate the inhibitory effects of CAPE on the invasive phenotype of SK-Hep1 cells.
Tumor cell invasion involves degradation of the basement membrane and the stromal ECM by members of the MMP family. Numerous studies have shown that the levels of MMP-2 and/or MMP-9 are associated with the invasive phenotype of tumor cells. An imbalance between proteinases and their inhibitors has been demonstrated to be a pivotal factor in carcinogenesis, particularly tumor progression. We previously suggested that MMP-2 and MMP-9 play an important role in SK-Hep1 cells [5]. The present study demonstrated that the chemopreventive CAPE inhibits the invasiveness of SK-Hep1 cells as well as downregulates MMP-2 and MMP-9.
In the study reported here, we demonstrated that CAPE inhibited SK-Hep1 cell invasion, suggesting overlapping pathways for regulation of ECM-degrading activities. CAPE effectively inhibited invasion and downregulate MMP-2 and MMP-9. Thus, this study presents MMP-2 and MMP-9 as potential target molecules for the anti-invasive and antimigrative activities of CAPE in SK-Hep1 cells. The promoter region of MMP-9 and MMP-2 genes has been shown to contain consensus motifs for NF-κB. We recently reported that CAPE significantly inhibited H-ras-induced NF-κB DNA-binding activity without affecting activation of ERK1/2 and p38, which are major intracellular molecules of ras-mediating signals [7]. The study reported here also demonstrates that CAPE strongly inhibits NF-κB DNA-binding activity in SK-Hep1 cells. Taken together, the results suggest that CAPE inhibits the invasive phenotype of SK-Hep1 cells through inhibition of MMP-2 and MMP-9 expression, which may be at least partly associated with blockade of NF-κB activation.
Acknowledgments
This work was supported by research grants from the Korea Institute of Science and Technology Evaluation and Planning for Functional Food Research and Development, Ministry of Science and Technology, Republic of Korea (no. 2007-01866).
Footnotes
Ki Won Lee and Nam Joo Kang contributed equally to this work.
References
- 1.Birkedal-Hansen H (1995) Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol 7:728–735 [DOI] [PubMed]
- 2.Borrelli F, Izzo AA, Di Carlo G, Maffia P, Russo A, Maiello FM, Capasso F, Mascolo N (2002) Effect of a propolis extract and caffeic acid phenethyl ester on formation of aberrant crypt foci and tumors in the rat colon. Fitoterapia 73:S38–S43 [DOI] [PubMed]
- 3.Dolcet X, Llobet D, Pallares J, Matias-Guiu X (2005) NF-kB in development and progression of human cancer. Virchows Arch 446:475–482 [DOI] [PubMed]
- 4.Ferlay J, Bray F, Pisani P, Parkin DM (2000) Cancer incidence, mortality and prevalence worldwide, vol. 1
- 5.Jung JW, Cho SD, Ahn NS, Yang SR, Park JS, Jo EH, Hwang JW, Aruoma OI, Lee YS, Kang KS (2006) Effects of the histone deacetylases inhibitors sodium butyrate and trichostatin A on the inhibition of gap junctional intercellular communication by H2O2- and 12-O-tetradecanoylphorbol-13-acetate in rat liver epithelial cells. Cancer Lett 241:301–308 [DOI] [PubMed]
- 6.Lee KW, Lee CY (2002) Vitamins, diet and cancer prevention. Am J Clin Nutr 75:1122–1123 [DOI] [PubMed]
- 7.Lee KW, Chun KS, Lee JS, Kang KS, Surh YJ, Lee HJ (2004) Inhibition of cyclooxygenase-2 expression and restoration of gap junction intercellular communication in H-ras-transformed rat liver epithelial cells by caffeic acid phenethyl ester. Ann N Y Acad Sci 1030:501–507 [DOI] [PubMed]
- 8.Lee SJ, Lee KW, Hur HJ, Chun JY, Kim SY, Lee HJ (2007) Phenolic phytochemicals derived from red pine (Pinus densiflora) inhibit the invasion and migration of SK-Hep1 human hepatocellular carcinoma cells. Ann N Y Acad Sci 1095:536–544 [DOI] [PubMed]
- 9.Natarajan K, Singh S, Burke TR, Grunberger D, Aggarwal BB (1996) Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA 93:9090–9095 [DOI] [PMC free article] [PubMed]
- 10.Sato H, Kita M, Seiki M (1993) v-Src activates the expression of 92-kDa type IV collagenase gene through the AP-1 site and the GT box homologous to retinoblastoma control elements. A mechanism regulating gene expression independent of that by inflammatory cytokines. J Biol Chem 268:23460–23468 [PubMed]
- 11.Shishodia S, Aggarwal BB (2004) Nuclear factor-kappaB activation mediates cellular transformation, proliferation, invasion angiogenesis and metastasis of cancer. Cancer Treat Res 119:139–173 [DOI] [PubMed]
- 12.Su ZZ, Lin J, Prewett M, Goldstein NI, Fisher PB (1995) Apoptosis mediates the selective toxicity of caffeic acid phenethyl ester (CAPE) toward oncogene-transformed rat embryo fibroblast cells. Anticancer Res 15:1841–1848 [PubMed]
- 13.Ura H, Bonfil RD, Reich R, Reddel R, Pfeifer A, Harris CC, Klein-Szanto AJ (1989) Expression of type IV collagenase and procollagen genes and its correlation with the tumorigenic, invasive, and metastatic abilities of oncogene-transformed human bronchial epithelial cells. Cancer Res 49:4615–4621 [PubMed]