Abstract
In this communication, we document the self-assembly of heterologously expressed truncated human aromatase (CYP19) into nanometer scale phospholipids bilayers (Nanodiscs). The resulting P450 CYP19 preparation is stable, and can tightly associate the substrate androstenedione to form a nearly complete high-spin ferric protein. Ferrous CYP19 in Nanodiscs was mixed anaerobically in a rapid-scan stopped-flow with atmospheric dioxygen and the formation of the ferrous-oxy complex observed. First order decay of the oxy-complex to release superoxide and regenerate the ferric enzyme was monitored kinetically. Surprisingly, the ferrous-oxy complex of aromatase is more stable that of hepatic CYP3A4, opening the path to precisely determine the biochemical and biophysical properties of the reaction cycle intermediates in this important human drug target.
Introduction
Steroid hormones are synthesized from cholesterol in a complex series of enzymatic steps [1]. Central to these processes, and deterministic in the paths to the major classes of androgens, estrogens and mineral corticoids is the regio- and stereo- specific action by a variety of cytochrome P450 (CYP) proteins. For instance, formation of the androgens requires attack at the 17-carbon to form a 17-keto group at the apex of the D ring while estrogens are formed by attack at the 19-carbon which leads to aromatization of the A-ring (see refs [2, 3] for general review). In humans, this latter reaction is catalyzed by the microsomal CYP19, and has emerged as an important pharmaceutical target for the treatment of estrogen-dependent breast cancers [4] as well as non-small cell lung carcinomas [5]
CYP19 is thought to catalyze three distinct reactions [6, 7]. First is hydroxylation of the 19-methyl via a common P450 mechanism involving generation of a higher valent oxo-complex at the heme iron [8], hydrogen abstraction and “oxygen rebound” [9, 10] to form the product primary alcohol. A second monoxygenase activity, stoichiometrically consuming one reduced pyridine nucleotide (NADPH) and atmospheric dioxygen generates a gem-diol, which collapses to the 19-aldehyde. The special activity of aromatase is the third step, which also utilizes atmospheric dioxygen and NADPH, resulting in the release of formaldehyde and aromatization of the A-ring [6, 7, 11]. The development of specific aromatase inhibitors would be greatly aided through understanding the detailed chemistry of this latter reaction.
P450 reaction chemistry has been the subject of intense research for over three decades. As illustrated in Figure 1, the catalytic cycle is envisioned to involve substrate binding to the ferric enzyme and one electron reduction of the heme followed by the binding of atmospheric dioxygen to form the ferrous-oxy state. This is the critical intermediate and branch-point in P450 mechanisms where the control of proton delivery by the enzyme defines the catalytic results. Following second electron input to form a peroxo-anion adduct, protonation of the distal oxygen leads to hydroperoxo formation and ultimate oxygen-oxygen bond scission to form the higher valent metal-oxo complex commonly termed “Compound I” [8, 12]. This pathway is thought to be operating in the first two steps of the aromatase reaction leading to the 19-aldehyde. The final step in aromatization of the A-ring, however, is much less well understood.
Figure 1.
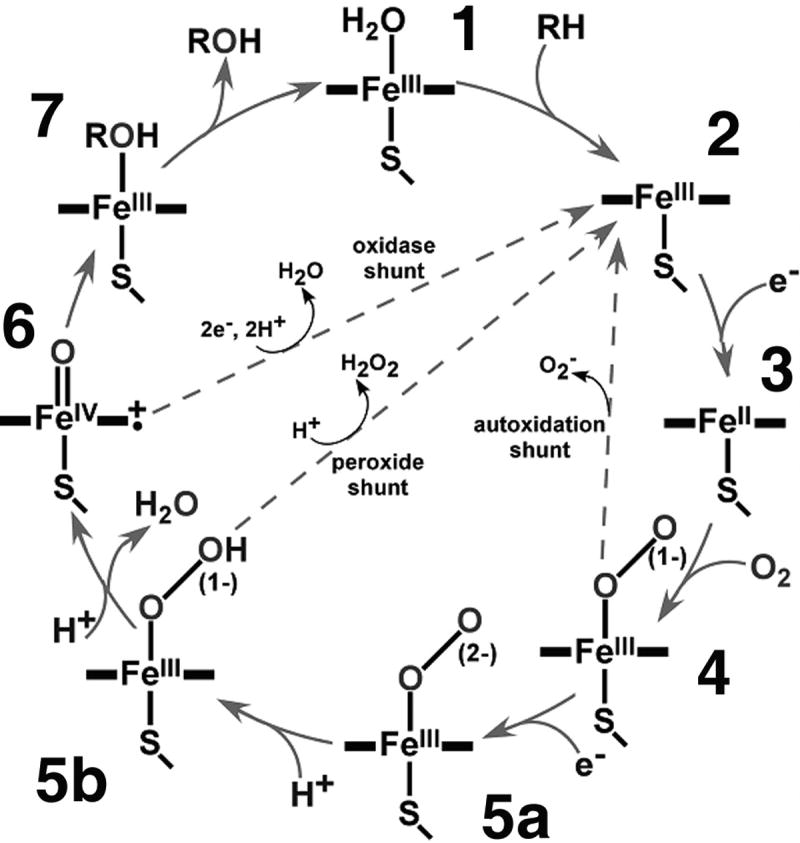
General scheme of the cytochrome P450 catalytic cycle. (Reproduced with permission from ref [7])
In 1982, Akhtar et al. first proposed [13] that the aromatization step involved not “Compound I” generation, but rather nucleophilic attack of the distal oxygen of peroxo-anion state on the electrophilic carbonyl of the 19-aldehyde, which is supported by a recent study of the human aromatase using resonance Raman spectroscopy [14]. Such reactivity was also observed in mutants of hepatic P450s [15] and has been proposed for the terminal step in nitric oxide synthase [16]. Clearly, critical features of the CYP19 active site are responsible for controlling the proton delivery process to define the processing of these oxygenated hemes.
The ferrous-oxy complex of a soluble bacterial P450, CYP101, was isolated and characterized three decades ago [17-20]. Defining such states in the membrane bound P450s was much more difficult due to aggregation, poor reactivity or instability in detergent or liposomes. Recently, we utilized the Nanodisc system to generate a self-assembled membrane architecture which is amiable to rapid reaction methodologies. Nanodisc incorporated reduced CYP3A4 were reacted with oxygen in stopped-flow, and the formation and decay of the ferrous-oxy complex determined [21, 22]. With this state defined, subsequent low-temperature stabilization and cryo-reduction could be used to generate the peroxo anion and hydroperoxo intermediates as demonstrated for CYP101. In order to begin dissecting the mechanistic action of human aromatase (CYP19), we undertook the challenge to document and characterize the autoxidation behavior of the ferrous-oxy complex in this more hydrophobic P450 system.
Materials and Methods
Truncated CYP19 was expressed in E.coli and purified as previously described [23]. Protein was in 250 mM KPi (pH 7.4) buffer containing 100 mM NaCl, 0.1 mM EDTA, 0.1 mM DTT, 1% (23 mM) sodium cholate and 1% (8 mM) Tween 20. The Nanodisc reconstitution mixture utilized the MSP1D1(-) membrane scaffold protein [24] at a concentration of 132 μM, POPC (8.3 mM) and cholate (16 mM) in 20 mM TrisCl (pH 7.4) containing 0.1 M NaCl. 100 μL of CYP19 solution was mixed with 200 μL of 50 mM potassium phosphate buffer (pH 7.4) containing 0.5 M sodium acetate, 2 mM DTT and 20 mM cholate together with 400 μL of the Nanodisc reconstitution mixture and incubated for three hours. The final molar ratios of CYP19:MSP:POPC were 1:14:800 in order to generate a single protein in the nanoscale bilayer in the presence of excess bare discs. Self-assembly was initiated by the addition of 0.6 g wet Biobeads™ and the sample placed on a rotary shaker for 2 hours. The sample was loaded onto a Ni-NTA affinity column using the histidine tag contained on the MSP, washed with buffer containing 15 mM imidazole and eluted with 300 mM imidazole.
CYP19 incorporated into Nanodiscs was analyzed by size exclusion chromatography on Superdex 200 as has been described in detail for CYP3A4 [25]. Figure 2 shows the elution profile monitored at 417 nm to follow the heme prosthetic group and 280 nm to monitor total protein (MSP+CYP19). CYP19 containing Nanodiscs run slightly larger than the bare discs. Nanodiscs containing both CYP19 and the rat NADPH-cytochrome P450 reductase were generated as previously described [26].
Figure 2.
Size exclusion chromatography of CYP19 incorporated in Nanodiscs, monitored using optical absorption at 280 nm (1, total protein) and 417 nm (2, CYP19), top bar shows calibration of the column using a standard set of proteins.
Results and Discussion
Despite the high hydrophobicity and instability of aromatase and the difficulty in obtaining high expression levels in heterologous systems, Figure 3 demonstrates the facile incorporation of CYP19 into Nanodiscs. The resulting P450 preparation showed no evidence of the inactive P420 form. In separate experiments wherein the rat NADPH reductase was co-incorporated with CYP19, a substrate dependent turnover of androstenedione demonstrated functional enzyme (60-65 nmol NADPH oxidized per minute per mg CYP19) (data not shown). The Nanodisc incorporated CYP19 is also significantly more stable, and can be stored at 4° C for many weeks without formation of inactive enzyme.
Figure 3.
Optical absorption spectra of CYP19 in Nanodiscs in the presence of different concentrations of androstenedione. The substrate free protein is low spin with a Soret maximum at 417 nm which shifts to the high-spin form characterized by the peak at 396 nm in the presence of 200 μM of the substrate.
CYP19 is active on a variety of steroids containing the 19-methyl group. The resting state of cytochrome P450 contains a heme prosthetic group in the Fe3+ ferric state with a sixth water ligand trans to the protein supplied thiolate. The iron is in the low-spin (S=1/2) state, characterized by a Soret absorbance near 417 nm. In many, if not most, cases substrate binding can tend to displace this distal bound water, reducing the ligand field strength thus allowing the iron to adopt a high-spin (S=5/2) configuration which can be monitored by a shift in the Soret maximum to ∼ 390 nm. The incorporation of membrane bound P450s into Nanodiscs has revealed that the mechanisms for substrates to perturb the spin equilibrium (and coupled redox potential) are similar to that operating in the soluble, microbial systems [27]. This spin-state shift has also been utilized as an easy probe of native activity. Substrate binding has also been shown to stabilize the ferrous-oxy complex of various cytochrome P450s [20, 21]. We determined the interaction of the substrate androstenedione with the ferric CYP19 in Nanodiscs. Figure 3 shows a clean “Type-I” binding spectra with increasing substrate concentrations, with ∼90% high-spin conversion at substrate saturation. A spectral dissociation constant, determined by fitting of the exact quadratic binding isotherm, yielded a value of Ks = 2 μM.
As previously noted, the ferrous-oxy complex of the cytochrome P450s is a central intermediate in catalysis. The local enzyme active site is responsible for control of proton delivery to the bound dioxygen molecule, which in the case of the normal oxygenase chemistry operating in the first two steps of CYP19, needs two protonations of the distal oxygen atom to cleave the O-O bond [28]. For the third step, however, current dogma, suggests that the distal oxygen is left unprotonated to allow nucleophilic attack on a 19-carbonyl intermediate. Understanding the details of aromatase catalysis requires the ability to isolate and characterize the CYP19 ferrous-oxy complex.
Using a androstenedione saturated CYP19-Nanodisc preparation and, taking advantage of the incredible ruggedness of Nanodiscs to biophysical manipulation, we mixed an anaerobic sample of the ferrous enzyme with oxygen saturated buffer in stopped-flow with optical monitoring via a rapid data processing diode array spectrophotometer. Within ∼ 25 msec, a characteristic Soret shift to 418 nm was observed, with the subsequent one-electron auto-oxidative decay [19] to form superoxide [18] and ferric enzyme (Figure 4). The spectral decay was fitted well by a single exponential with a rate constant of 0.21 s-1 at 25° C and 0.7 s-1 at 37° C. Importantly, and enabling for in-progress experiments to document the peroxo anion and hydroperoxo states, the ferrous oxy intermediate was found to be several fold more stable than that in the hepatic CYP3A4 [21].
Figure 4.

Decay of the CYP19 oxy-complex is monitored by optical absorption spectroscopy. Shown are the spectral changes of oxygenated CYP19 measured at 25° C from 20 ms (Soret maximum of the oxygenated form at 418 nm) to 15 s (ferric heme Soret maximum at 396 nm). Inset: Kinetics of autoxidation fitted with a single exponential.
In summary, we have demonstrated that human P450 CYP19 (aromatase) can be easily self-assembled into Nanodiscs, offering an active, stable, soluble and robust entity. The membrane embedded CYP19 binds androstenedione tightly, with over 90% conversion to the high spin form of the enzyme. Using stopped-flow rapid scan spectrophotometry, we were able to document the formation and decay of the critical ferrous-oxygen bound state for the first time. This intermediate is found to be more stable to one-electron autoxidation than the hepatic CYP3A4, offering the opportunity to understand the detailed chemical and biophysical properties of the aromatase catalytic cycle, an important pharmaceutical target for estrogen-dependent breast cancers and non-small cell lung carcinomas.
Acknowledgments
We acknowledge helpful discussions with and continuing experimental work by Dr. Stephanie Gantt in our laboratory. This work is supported by grants from the National Institutes of Health (GM31756 and GM33775 to S.G.S; GM69970 and ES00267 to M.R.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kagawa N, Bischof LJ, Cheng PY, Anwar A, Waterman MR. Biochemical diversity of peptide-hormone-dependent regulation of steroidogenic P450s. Drug Metab Rev. 1999;31:333–342. doi: 10.1081/dmr-100101921. [DOI] [PubMed] [Google Scholar]
- 2.Hakki T, Bernhardt R. CYP17- and CYP11B-dependent steroid hydroxylases as drug development targets. Pharmacol Ther. 2006;111:27–52. doi: 10.1016/j.pharmthera.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Ghayee HK, Auchus RJ. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord. 2007;8:289–300. doi: 10.1007/s11154-007-9052-2. [DOI] [PubMed] [Google Scholar]
- 4.Brueggemeier RW, Hackett JC, Diaz-Cruz ES. Aromatase inhibitors in the treatment of breast cancer. Endocr Rev. 2005;26:331–345. doi: 10.1210/er.2004-0015. [DOI] [PubMed] [Google Scholar]
- 5.Oyama T, Sugio K, Isse T, Matsumoto A, Nose N, Uramoto H, Nozoe T, Morita M, Kagawa N, Osaki T, Muto M, Yasumoto K, Kawamoto T. Expression of cytochrome P450 in non-small cell lung cancer. Frontiers Biosci. 2008;13:5787–5793. doi: 10.2741/3116. [DOI] [PubMed] [Google Scholar]
- 6.Akhtar M, Wright JN. A unified mechanistic view of oxidative reactions catalysed by P-450 and related Fe-containing enzymes. Nat Prod Rep. 1991;8:527–551. doi: 10.1039/np9910800527. [DOI] [PubMed] [Google Scholar]
- 7.Akhtar M, Lee-Robichaud P, Akhtar ME, Wright JN. The impact of aromatase mechanism on other P450s. J Steroid Biochem Mol Biol. 1997;61:127–132. [PubMed] [Google Scholar]
- 8.Denisov IG, Makris TM, Sligar SG, Schlichting I. Structure and chemistry of cytochrome P450. Chem Rev. 2005;105:2253–2278. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 9.Groves JT, McClusky GA. Aliphatic hydroxylation via oxygen rebound. oxygen transfer catalyzed by iron. J Am Chem Soc. 1976;98:859–861. [Google Scholar]
- 10.Watanabe Y, Groves JT. Molecular mechanism of oxygen activation by P-450. In: Sigman DS, editor. The Enzymes. Vol. 20. Academic Press; San Diego: 1992. pp. 405–452. [Google Scholar]
- 11.Hackett JC, Brueggemeier RW, Hadad CM. The final catalytic step of cytochrome p450 aromatase: a density functional theory study. J Am Chem Soc. 2005;127:5224–5237. doi: 10.1021/ja044716w. [DOI] [PubMed] [Google Scholar]
- 12.Groves JT, Han Y. Models and mechanisms of cytochrome P450 action. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Function, Genetics. Kluwer Academic/Plenum Publishers; New York: 2005. pp. 1–43. [Google Scholar]
- 13.Akhtar M, Calder MR, Corina DL, Wright JN. Mechanistic studies on C-19 demethylation in oestrogen biosynthesis. Biochem J. 1982;201:569–580. doi: 10.1042/bj2010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tosha T, Kagawa N, Ohta T, Yoshioka S, Waterman MR, Kitagawa T. Raman evidence for specific substrate-induced structural changes in the heme pocket of human cytochrome P450 aromatase during the three consecutive oxygen activation steps. Biochemistry. 2006;45:5631–5640. doi: 10.1021/bi060094a. [DOI] [PubMed] [Google Scholar]
- 15.Roberts ES, Vaz AD, Coon MJ. Catalysis by cytochrome P-450 of an oxidative reaction in xenobiotic aldehyde metabolism: deformylation with olefin formation. Proc Nat Acad Sci USA. 1991;88:8963–8966. doi: 10.1073/pnas.88.20.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuehr DJ, Wei CC, Wang Z, Hille R. Exploring the redox reactions between heme and tetrahydrobiopterin in the nitric oxide synthases. Dalton Trans. 2005:3427–3435. doi: 10.1039/b506355h. [DOI] [PubMed] [Google Scholar]
- 17.Peterson JA, Ishimura Y, Griffin BW. Pseudomonas putida cytochrome P-450: Characterization of an oxygenated form of the hemoprotein. Arch Biochem Biophys. 1972;149:197–208. doi: 10.1016/0003-9861(72)90315-3. [DOI] [PubMed] [Google Scholar]
- 18.Sligar SG, Lipscomb JD, Debrunner PG, Gunsalus IC. Superoxide anion production by the autoxidation of cytochrome P450cam. Biochem Biophys Res Commun. 1974;61:290–296. doi: 10.1016/0006-291x(74)90565-8. [DOI] [PubMed] [Google Scholar]
- 19.Lipscomb JD, Sligar SG, Namtvedt MJ, Gunsalus IC. Autooxidation and hydroxylation reactions of oxygenated cytochrome P-450cam. J Biol Chem. 1976;251:1116–1124. [PubMed] [Google Scholar]
- 20.Eisenstein L, Debey P, Douzou P. P 450cam: oxygenated complexes stabilized at low temperature. Biochem Biophys Res Comm. 1977;77:1377–1383. doi: 10.1016/s0006-291x(77)80131-9. [DOI] [PubMed] [Google Scholar]
- 21.Denisov IG, Grinkova YV, Baas BJ, Sligar SG. The ferrous-dioxygen intermediate in human cytochrome P450 3A4. Substrate dependence of formation and decay kinetics. J Biol Chem. 2006;281:23313–23318. doi: 10.1074/jbc.M605511200. [DOI] [PubMed] [Google Scholar]
- 22.Denisov IG, Grinkova YV, McLean MA, Sligar SG. The one electron autoxidation of human cytochrome P450 3A4. J Biol Chem. 2007;282:26865–26873. doi: 10.1074/jbc.M704747200. [DOI] [PubMed] [Google Scholar]
- 23.Kagawa N, Hori H, Waterman MR, Yoshioka S. Characterization of stable human aromatase expressed in E. coli. Steroids. 2004;69:235–243. doi: 10.1016/j.steroids.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J Am Chem Soc. 2004;126:3477–3487. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 25.Baas BJ, Denisov IG, Sligar SG. Homotropic cooperativity of monomeric cytochrome P450 3A4 in a nanoscale native bilayer environment. Arch Biochem Biophys. 2004;430:218–228. doi: 10.1016/j.abb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Denisov IG, Baas BJ, Grinkova YV, Sligar SG. Cooperativity in cytochrome P450 3A4: linkages in substrate binding, spin state, uncoupling, and product formation. J Biol Chem. 2007;282:7066–7076. doi: 10.1074/jbc.M609589200. [DOI] [PubMed] [Google Scholar]
- 27.Das A, Grinkova YV, Sligar SG. Redox potential control by drug binding to cytochrome P450 3A4. J Am Chem Soc. 2007;129:13778–13779. doi: 10.1021/ja074864x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loew GH, Harris DL. Role of the heme active site and protein environment in structure, spectra, and function of the cytochrome P450s. Chem Rev. 2000;100:407–419. doi: 10.1021/cr980389x. [DOI] [PubMed] [Google Scholar]




