Abstract
Working under the assumption that hormesis is triggered by specific types of DNA damage, this report focuses on the types of damage which form the signature of ionizing radiation. The key attribute of the signature is the clustering of damage, arising from clusters of energy deposition such that more than one site within a 10 base pair segment of DNA has been chemically altered. A brief overview is given on what is currently believed to be the primary components of clustered damage produced by the direct effect. The overview draws primarily on studies that utilize electron paramagnetic resonance to measure free radical intermediates and gel electrophoresis to measure clustered damage in plasmid DNA. Based on this information, the threshold for a radiation induced biological response is calculated.
Keywords: DNA damage, Direct effect, Plasmid DNA, Radiation chemistry, Radiation products
INTRODUCTION
Underlying any biological response to ionizing radiation there must be chemical changes. Because DNA is the critical cellular target (von Sonntag 2006), it is most likely that it is DNA damage that serves to trigger a response. More specifically, because clustered DNA damage is the unique attribute that makes ionizing radiation of particular importance in biology and medicine, the dose threshold for observing a biological response, and perhaps hormesis, should correlate with yields of clustered DNA damage. In this paper we give an overview of what is currently understood about the radiation physics and chemistry of DNA and use that information to estimate the threshold for a biological response.
The evidence continues to grow in support of the proposal by Ward (Ward 1981) and Goodhead (Goodhead 1990) that the main biological impact of ionizing radiation is that it creates lesions in DNA in the form of clusters. A lesion located near to others, i.e., in a cluster, is more prone to misrepair and is more likely to destabilize the genome (Shikazono, et al. 2006; Wallace, et al. 2004; Weinfeld, et al. 2001). Predicting biological response from first principles requires a quantitative understanding of the types of lesions formed and their spatial distribution. The degree to which lesions are clustered is a consequence of two factors, (i) the non-homogeneous deposition of energy that results in a track of initially altered sites, and (ii) the subsequent expansion of the track by diffusion of these altered sites. The indirect effect, a consequence of reactions between DNA and radicals generated in unbound water, requires diffusion of hydroxyl radicals from the water phase to the DNA target (see Figure 1). In contrast, the damage due to (what is loosely called) the direct effect, comes from precursors created by ionization of DNA and its solvent shell. The mean diffusion distance of these precursors is very short (Spalletta and Bernhard 1992; Yan, et al. 1992), < 1 nm (i.e., less than 3 base pairs) and consequently the damage has a high probability of being clustered. The direct effect, therefore, plays a major role in the fact that an imprint of the track is left on DNA (Nikjoo, et al. 2001).
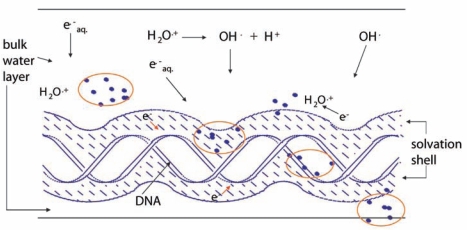
FIGURE 1.
DNA in aqueous solution. Cartoon showing the intersection of an ionizing radiation track with DNA, the solvation shell of DNA, and the bulk water in the vicinity of DNA. The solid dots represent energy deposition events, i.e., ionization and excitation. Ionization of bulk water yields the water radical cation, H2O+•, and the aqueous electron, e−aq, which may react with DNA giving indirect-type damage. Ionization of DNA and its solvation shell results in direct-type damage. The DNA solvation shell consists of up to ~20 to 22 waters/nucleotide.
The indirect effect of ionizing radiation was considered in the early radiobiology literature (von Sonntag 2006) to be more biologically relevant than the direct effect. Based on current knowledge, however, it is more likely that the direct effect is of comparable or even greater importance. Direct-type damage is formed via two routes. One is by direct ionization of the DNA and the other is by ionization of that portion of the solvent shell that is tightly bound to the DNA. Although the later is physically an indirect effect, by transferring holes and electrons created in the DNA solvation shell to the DNA, the initial DNA lesions are indistinguishable from those formed directly in the DNA. Damage formed via electron or hole transfer from the solvation shell has been termed, therefore, the “quasi-direct” effect (Becker and Sevilla 1993). The damage created by both routes is called collectively “direct-type” damage (Milano and Bernhard 1999), distinguishing it from “indirect-type” damage that is due to reactions with hydroxyl radicals and aqueous electrons. Proof and quantification of damage transfer to DNA from its solvation shell (Becker, et al. 1997; Debije, et al. 2000; Milano and Bernhard 1999) has proved quite important. Inclusion of the solvation shell effectively doubles the target mass resulting in direct-type damage. This doubling is used in the calculation reported in the final section of this paper. The fact that the solvation shell magnifies direct-type damage, and its importance in the formation of clustered damage, is not yet fully appreciated.
There are good reasons to believe that the composition of clusters is dominated by direct-type damage. It is often quoted that the direct effect accounts for between 30% and 50% of the damage in vivo. As reviewed by von Sonntag (von Sonntag 2006), it is difficult to design experiments capable of dividing the direct effect from the indirect effect; this is particularly true for in vivo measurements. In our view, one of the better estimates of the relative contribution of the direct-effect is that by Krisch, et al. (Krisch, et al. 1991). Using a well defined system consisting of SV40 viral DNA and varying the scavenger efficiency by over four orders of magnitude, they measured ssb and dsb with gel electrophoresis. Moreover, they compared the yields of ssb/dsb at −75 °C (where the direct effect predominates) with those at 0–20 °C. They estimated that under physiological conditions ~50% of the damage would be due to the direct effect. This percentage was based on only one type of damage, the deoxyribose damage that results in ssb and dsb. From our recent studies (Purkayastha, et al. 2007), less than 40% of the clustered lesions are of the type that would be revealed as dsb in the Krisch experiments. In addition, we have shown that damage at C1′ of the deoxyribose is not readily revealed as an ssb (Roginskaya, et al. 2005a; Roginskaya, et al. 2005b), and it would have most likely been missed in these experiments. If experiments were designed to take these two observations into account, the likely outcome would be that the lesions making up clusters are predominantly formed from direct-type damage. These estimates pertain to low LET radiation; at high LET, direct-type damage would account for an even larger fraction of the clustered damage(Becker, et al. 1996; Nikjoo, et al. 1998; Roots, et al. 1990; Yokoya, et al. 2003).
That direct-type damage accounts for a significant fraction of all of the damage to DNA in vivo should not be surprising. In eukaryotes, DNA is tightly packed within chromatin, conferring upon it mixed properties of the aqueous state and solid state. Measurements of the concentration of DNA in the nucleus over a wide range of eukaryotic cells, give an average of ~100 mol H2O/mol nucleotide (Daban 2000). Given that: (i) 10% of this water is an integral part of the DNA target, (ii) some fraction (~50%) is part of the protein solvation shell, and (iii) the very high protein concentration scavenges a large fraction of the HO•, one would anticipate that the direct effect accounts for about half of the DNA damage and more than half of the clustered damage.
REACTION PATHWAYS LEADING TO CLUSTER FORMATION BY THE DIRECT EFFECT
Clusters are formed when secondary electrons reach energies low enough that electrons created by further inelastic collisions undergo addition collisions in close proximity to one another. In the track structure field, these are called spurs and blobs (Magee and Chatterjee 1978). Once thermalized, the samples contain a non-homogeneous distribution of ionized sites. The track consists of trapped ions and electronically excited molecules. With respect to DNA damage, excited states appear to be negligible (Bernhard, et al. 1994). The trapped ions consist of one-electron-loss sites (free radical cations) and one-electron-gain sites (free radical anions). We refer to these as holes and electron-gain sites, respectively. These free radicals are inherently unstable, reacting quickly at room temperature to give stable diamagnetic damage which cannot be detected by electron paramagnetic resonance (EPR) spectroscopy. The challenges posed by the instability of free radical intermediates have been met by studying radicals trapped in solid state DNA at low temperatures using EPR.
There is without question more than one type of reaction pathway giving rise to direct-type damage. The pathway presented here is one that we believe is likely to account for the largest fraction of end-product. We call it the trappable-radical single-track pathway (Swarts, et al. 2007); other pathways are mentioned below. The trappable-radical single-track pathway is one in which the initial radicals, formed by either electron loss or electron gain, escape the spur of ionizations. These isolated radicals can be trapped, their structures can be analyzed by cryogenic EPR, and the thermally driven reactions can be studied by heating. Eventually, the radicals form stable end-product in which all electrons are paired. These diamagnetic end-products can no longer be detected by EPR; other analytical techniques must be applied.
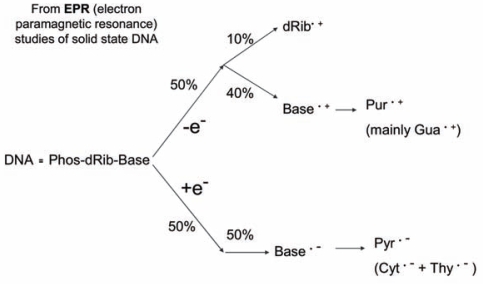
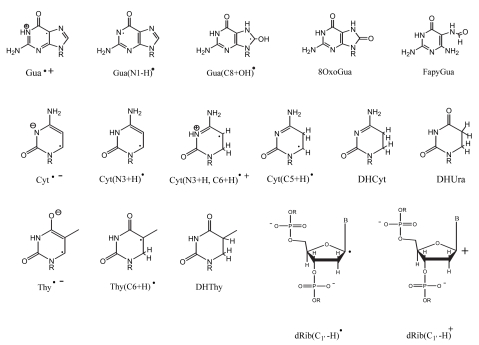
Our mechanistic model assumes that half of the radicals trapped at 4 K are derived from sites of electron loss, holes, and the other half from sites of one-electron gain (Figure 2). Within a few picoseconds of the energy deposition, the system is thermalized, and the electrons are captured exclusively by the bases. The holes, meanwhile, are initially formed on the sugar-phosphate backbone, the DNA solvation shell, and the bases. Holes, initially formed in that portion of the solvation shell directly bound to DNA, transfer to the sugar and bases (Becker, et al. 1997; Debije, et al. 2000). The four predominant radical types are dRib• (deoxyribose radical), Gua+• (guanine radical cation), Cyt−• (cytosine radical anion), and Thy−• (thymine radical anion). Their relative concentrations are about 1:4:4:1, respectively (Bernhard 1989; Bernhard and Close 2003; Purkayastha, et al. 2006). The structures of the free radical intermediates, stable end products, and corresponding labels are given in Figure 3. The radicals, with the exception of Thy−•, are unstable at 4 K and are not observed by EPR. By undergoing dielectric relaxation, consisting primarily of proton transfer to the radical anions and proton transfer away from the radical cations, the radicals are stabilized and readily studied by EPR (Debije and Bernhard 2002).
FIGURE 2.
Initial radical ion distribution. A model showing the distribution of radical ions initially formed by direct ionization of DNA. The model is based primarily on EPR experiments. Further detail is given in Figures 4–6 and chemical structures are given in Figure 3.
FIGURE 3.
Chemical structures and notation used in the text.
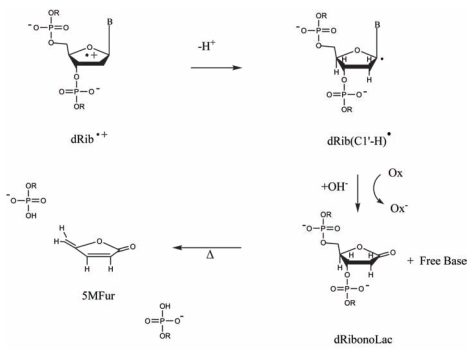
The thermally activated reactions stemming from dRib• depend on which deoxyribose carbon has undergone the net loss of a hydrogen atom. In Figure 4, the reaction pathway for the C1′ centered radical, dRib(C1′-H)• is given as a specific example. One-electron oxidation of the radical followed by OH− addition releases free base and leaves behind an intact DNA backbone containing deoxyribonolactone (dRibonoLac). The deoxyribonolactone lesion is relatively stable at room temperature; but, upon warming, it yields 5-methylenefuranone and a cleaved strand (Roginskaya, et al. 2005a; Roginskaya, et al. 2005b).
FIGURE 4.
The deoxyribose radical cation, dRib+•, deprotonates from any of the five deoxyribose carbons. Shown here is an example of the reactions stemming from C1′ deprotonation.
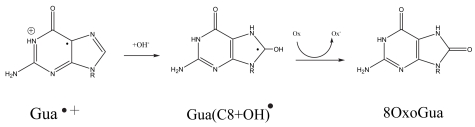
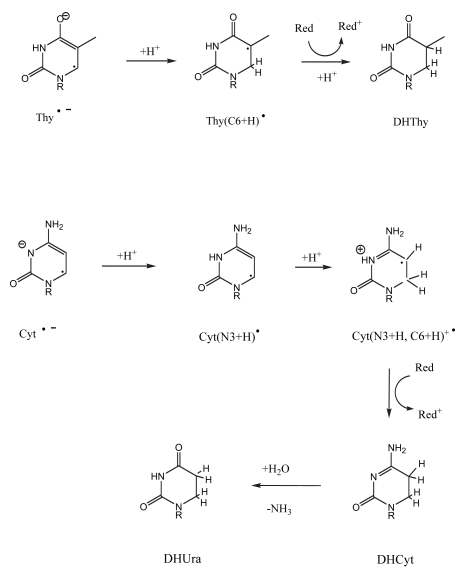
One of the major pathways initiated by one-electron oxidation of guanine is shown in Figure 5. The guanine radical cation, G+•, undergoes OH− addition creating the neutral G(C8+OH)• radical (Cullis, et al. 1996). Subsequent one-electron oxidation gives the well known product of 8-oxoGua. The fate of one-electron reduced thymine, Thy−•, is shown in Figure 6. It protonates at C6 to give the neutral Thy(C6+H)• radical (Wang, et al. 1997; Wang and Sevilla 1994), which when one-electron reduced results in the formation of 5,6-dihydrothymine, DHThy. The type of reaction sequence produces 5,6-dihydrocytosine from cytosine (Debije, et al. 2002), but in this case hydrolysis leads to deamination and the final stable end product is 5,6-dihydrouracil, DHUra.
FIGURE 5.
The reaction pathway leading to the formation of 8-oxo-guanine starting from the guanine radical cation, Gua+•.
FIGURE 6.
The reaction pathways leading to 5,6-dihydrothymine, DHThy, and 5,6-dihydrouracil, DHUra, starting from the respective one-electron reduced pyrimidines, Thy−• and Cyt−•.
By this model, four of the major lesions produced in DNA by the direct effect are explained. The four lesions are strand breaks, 8-oxoGua, diHUra, and diHThy (Swarts, et al. 2007).
The above model is not all inclusive. There are other mechanisms and other DNA products. The above pathway is initiated by inelastic collisions between the electron and DNA. Other pathways exist; an important set of reactions are those initiated by the elastic scattering of low energy electrons (LEE). LEE have been shown to undergo dissociative electron attachment (DEA) to DNA, producing single strand breaks (ssb) and double strand breaks (dsb), as well as fragmented bases (Huels, et al. 2003; Huels, et al. 1998; Li, et al. 2006). The yield of these end points, however, is not known. Ionization of the deoxyribose moiety can occur indirectly via the purines. It has been recently discovered that the guanine radical cation, when photoexcited by 520 nm light, undergoes hole transfer to the deoxyribose (Adhikary, et al. 2006; Adhikary, et al. 2005). The net result is a selective loss of hydrogen from C1′, C3′, and C5′ of the deoxyribose. Furthermore, when radical cations are formed by direct ionization, they are initially left in an electronic excited state. Thus, it has been proposed that this reaction may occur in DNA due to the direct effect of ionizing radiation (Adhikary, et al. 2006). Swarts and co-workers observed a further 12 oxidation products in addition to those discussed above, for example 8-oxo-adenine (8-oxoAde), 5-OH-cytosine, and 5-OH-uracil (Swarts, et al. 1996). Molecular hydrogen has been measured as a function of hydration level (Falcone, et al. 2005). Interstrand crosslinks have been observed in halogenated DNA containing single stranded segments (Cai, et al. 2005).
PREDICTED THRESHOLD FOR A BIOLOGICAL RESPONSE
In recent work (Purkayastha, et al. 2007), we measured the yields of clustered lesions in DNA. The yield of DNA base damages due to the direct-type effects of ionizing radiation was compared with the yield of DNA trapped radicals previously measured in the same pUC18 plasmid across the hydration range of 2.5 to 22.2 mol water per mol nucleotide. Single strand breaks, ssb, and double strand breaks, dsb, were detected by agarose gel electrophoresis. Specific types of base lesions were converted into ssb and dsb using the base-excision repair enzymes, Endo III and FPG. The yields of dsb were consistent with changes in free radical trapping as a function of hydration. The composition of three types of clusters and the yields of each cluster type was determined. The yield of dsb due to deoxyribose damage on opposing strands was 3.5 ± 0.5 nmol/J for fully hydrated pUC18. For an oxidized purine or an abasic site on one strand and an oxidized purine, an abasic site, or a deoxyribose damage on the opposite strand, the yield was 3.2 ± 0.9 nmol/J. For a reduced pyrimidine or an abasic site on one strand and a reduced pyrimidine, an abasic site, or a deoxyribose damage on the opposite strand, the yield was >2.3 ± 0.9 nmol/J, where the inequality reflects the possibility that reductive damage is underreported in the protocol employed. Less than 40% of the clustered lesions are due to opposing deoxyribose damage, the damage that would be scored as prompt dsb. The yield for these three types of clusters is ~10 nmol/J; this yield is employed in the following calculation.
The 46 human chromosomes are comprised of 7.8×109 bp, giving a molecular weight of 5.2×1012 Da assuming one Na+ per nucleotide. Because the hydration shell transfers direct damage into the DNA (see above) there are ~22 waters per nucleotide that are part of the target mass. With respect to direct damage, the target mass is, therefore, ~11×1012 Da. Using 10 nmol/J and a dose of 1 mGy, the experimentally obtained yield (for what are presumed to be three of the major types of clusters produced by the direct effect) is ~ 1 cluster per 10 genomes. If damage to 1% of the cells is required to detect a biological response, then a threshold dose of ~0.1 mGy is predicted. This estimate is based on clusters formed solely by the direct effect. If we assume that inclusion of indirect DNA damage doubles the yield of clusters, then the predicted threshold is ~0.05 mGy.
In concluding, we compare these predicted levels of clustered damage with measured damage and damage responses in vivo. DSB were measured in human fibroblasts using immunofluorescence to detect γ-H2AX foci at very low doses of 90 kV x-rays (Rothkamm and Löbrich 2003). They found that a 1 mGy exposure resulted in 1 DSB per ~30 cells. If we assume that DSB account for 40% of the clustered damage, as per the results described above, this corresponds to 1 clustered lesion per ~ 10 cells; the same as predicted for cluster formation by the direct effect alone.
The yield of chromosomal aberrations is of interest because they are detected subsequent to substantial biochemical processing. In primary human fibroblasts exposed to 1 Gy of γ-rays, one aberration is detected per 20 cells (Cornforth, et al. 2002). It seems rather certain, therefore, that by the time chromosomal aberrations can be scored, the genome has sustained a level of clustered damage that is about three orders of magnitude larger than the number of aberrations. Better insight into the correspondence between clustered damage and biological endpoints, should be possible by finding models that are more sensitive to radiation exposure.
An example of a highly sensitive model is recombination mutagenesis in pKZ1 transgenic mice (Matsuoka, et al. 1991). Hooker, et al. used this model to study gene inversion in the spleen at very low doses of 250 kV X-rays administered to pKZ1 mice (Hooker, et al. 2004). They found a statistically significant gene inversion response of ~2×10−4 per cell at 5 μGy while the response at 1 μGy was not significant. Based on our calculated threshold and given that the size of mouse genome is ~86% that of the human genome, at 5 μGy the frequency of clustered damage is predicted to be ~5×10−4 per cell. The prediction, thereby, is that one gene inversion is produced for every ~2 cells that have sustained clustered damage. This implies that induction of recombinatorial repair is exceptionally sensitive to the presence of clustered damage or, as mentioned by Hooker, et al., that a bystander effect (Mothersill and Seymour 2001) may amplify the response.
ACKNOWLEDGMENT
This study was supported by PHS grants 2-R01-CA32546 (to WAB), and 2-R01-CA46295 (to JRM), awarded by the National Cancer Institute, DHHS.
REFERENCES
- Adhikary A, Kumar A, Sevilla MD. Photo-induced hole transfer from base to sugar in DNA: relationship to primary radiation damage. Radiat Res. 2006;165:479–484. doi: 10.1667/rr3563.1. [DOI] [PubMed] [Google Scholar]
- Adhikary A, Malkhasian AYS, Collins S, Koppen J, Becker D, Sevilla MD. UVA-visible photo-excitation of guanine radical cations produces sugar radicals in DNA and model structures. Nucl Acid Res. 2005;33:5553–5564. doi: 10.1093/nar/gki857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Razskazovskii Y, Callaghan MU, Sevilla MD. Electron spin resonance of DNA irradiated with a heavy-ion beam (16O8+): evidence for damage to the deoxyribose phosphate backbone. Radiat Res. 1996;146:361–368. [PubMed] [Google Scholar]
- Becker D, Sevilla MD. The chemical consequences of radiation damage to DNA. In: Lett JT, Sinclair WK, editors. Advances in Radiation Biology. Academic Press; San Diego: 1993. pp. 121–180. [Google Scholar]
- Becker D, Sevilla MD, Wang W, LaVere T. The role of waters of hydration in direct-effect radiation damage to DNA. Radiat Res. 1997;148:508–510. [Google Scholar]
- Bernhard WA. Sites of electron trapping in DNA as determined by ESR of one-electron reduced oligonucleotides. J Phys Chem. 1989;93:2187–9. [Google Scholar]
- Bernhard WA, Close DM. DNA damage dictates the biological consequence of ionizing radiation: the chemical pathways. In: Mozumder A, Hatano Y, editors. Charged particle and photon interactions with matter. Marcel and Dekker; New York: 2003. pp. 471–489. [Google Scholar]
- Bernhard WA, Mroczka N, Barnes J. Combination is the dominant free radical process initiated in DNA by ionizing radiation: an overview based on solid-state EPR studies. Int J Radiat Biol. 1994;66:491–7. doi: 10.1080/09553009414551511. [DOI] [PubMed] [Google Scholar]
- Cai Z, Cloutier P, Sanche L, Hunting D. DNA interduplex crosslinks induced by Al(Kalpha) X rays under vacuum. Radiat Res. 2005;164:173–179. doi: 10.1667/rr3408. [DOI] [PubMed] [Google Scholar]
- Cornforth MN, Bailey SM, Goodwin EH. Dose responses for chromosome aberrations produced in noncycling primary human fibroblasts by alpha particles, and by gamma rays delivered at sublimiting low dose rates. Radiat Res. 2002;158:43–53. doi: 10.1667/0033-7587(2002)158[0043:drfcap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cullis PM, Malone ME, Merson-Davies LA. Guanine radical cations are precursors of 7,8-dihydro-8-oxo-2′-deoxyguanosine but are not precursors of immediate strand breaks in DNA. J Am Chem Soc. 1996;118:2775–81. [Google Scholar]
- Daban JR. Physical constraints in the condensation of eukaryotic chromosomes. Local concentraion of DNA versus linear packing ratio in higher order chromatin structures. Biochemistry. 2000;39:3861–3866. doi: 10.1021/bi992628w. [DOI] [PubMed] [Google Scholar]
- Debije MG, Bernhard WA. Thermally stable sites for electron capture in directly ionized DNA: free radicals produced by the net gain of hydrogen at C5/C6 of cytosine and thymine in crystalline oligodeoxynucleotides. J Phys Chem A. 2002;106:4608–4615. [Google Scholar]
- Debije MG, Close DM, Bernhard WA. Reductive damage in directly ionized DNA: saturation of the C5=C6 bond of cytosine in d(CGCG)2 crystals. Radiat Res. 2002;157:235–242. doi: 10.1667/0033-7587(2002)157[0235:rdidid]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debije MG, Strickler MD, Bernhard WA. On the efficiency of hole and electron transfer from the hydration layer to DNA: an EPR study of crystalline DNA X-irradiated at 4 K. Radiat Res. 2000;154:163–170. doi: 10.1667/0033-7587(2000)154[0163:oteoha]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone JM, Becker D, Sevilla MD, Swarts SG. Products of the reactions of the dry and aqueous electron with hydrated DNA: hydrogen and 5,6-dihydropyrimidines. Radiat Phys and Chem. 2005;72:257–264. [Google Scholar]
- Goodhead DT. Radiation effects in living cells. Can J Phys. 1990;68:872–86. [Google Scholar]
- Hooker AM, Bhat M, Day TK, Lane JM, Swinburne SJ, Morley AA, Sykes PM. The linear no-threshold model does not hold for low-dose ionizing radiation. Radiat Res. 2004;162:447–452. doi: 10.1667/rr3228. [DOI] [PubMed] [Google Scholar]
- Huels MA, Boudaïffa B, Cloutier P, Hunting D, Sanche L. Single, double, and multiple double strand breaks induced in DNA by 3–100 eV electrons. J Am Chem Soc. 2003;125:4467–4477. doi: 10.1021/ja029527x. [DOI] [PubMed] [Google Scholar]
- Huels MA, Hahndorf I, Illenberger E, Sanche L. Resonant dissociation of DNA bases by subionization electrons. J Chem Phys. 1998;108:1309–1312. [Google Scholar]
- Krisch RE, Flick MB, Trumbore CN. Radiation chemical mechanisms of single– and double–strand break formation in irradiated SV40 DNA. Radiat Res. 1991;126:251–259. [PubMed] [Google Scholar]
- Li X, Sanche L, Sevilla MD. Base release in nucleosides induced by low-energy electrons: a DFT study. Radiat Res. 2006;165:721–729. doi: 10.1667/RR3568.1. [DOI] [PubMed] [Google Scholar]
- Magee JL, Chatterjee A. Theory of the chemical effects of high-energy electrons. J Phys Chem. 1978;82:2219–26. [Google Scholar]
- Matsuoka M, Nagawa F, Okazaki K, Kingsbury L, Yoshida K, Muller U, Larue DT, Winer JA, Sakano H. Detection of somatic DNA recombination in the trangenic mouse brain. Science. 1991;254:81–86. doi: 10.1126/science.1925563. [DOI] [PubMed] [Google Scholar]
- Milano MT, Bernhard WA. The influence of packing on free radical yields in solid-state DNA: film compared to lyophilized frozen solution. Radiat Res. 1999;152:196–201. [PMC free article] [PubMed] [Google Scholar]
- Mothersill C, Seymour C. Radiation-induced bystander effects: past history and future directions. Radiat Res. 2001;155:759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nikjoo H, O’Neill P, Wilson WE, Goodhead DT. Computational approach for determining the spectrum of DNA damage by ionising radiation. Radiat Res. 2001;156:577–583. doi: 10.1667/0033-7587(2001)156[0577:cafdts]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nikjoo H, Uehara S, Wilson WE, Hoshi M, Goodhead DT. Track structure in radiation biology: theory and applications. Int J Radiat Biol. 1998;73:355–364. doi: 10.1080/095530098142176. [DOI] [PubMed] [Google Scholar]
- Purkayastha S, Milligan JR, Bernhard WA. The role of hydration in the distribution of free radical trapping in directly ionized DNA. Radiat Res. 2006;166:1–8. doi: 10.1667/RR3585.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha S, Milligan JR, Bernhard WA. On the chemical yield of base lesions, strand breaks, and clustered damage generated in plasmid DNA by the direct effect of X-rays. Radiat Res. 2007;168:357–366. doi: 10.1667/RR0964.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roginskaya M, Bernhard WA, Marion RT, Razskazovskiy Y. The release of 5-methylene-2-furanone from irradiated DNA catalyzed by cationic polyamines and divalent metal cations. Radiat Res. 2005a;163:85–89. doi: 10.1667/rr3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roginskaya M, Razskazovskiy Y, Bernhard WA. 2-deoxyribonolactone lesions in X-ray-irradiated DNA: Quantitative determination by catalytic 5-methylene-2-furanone release. Angew Chem, Int Ed. 2005b;44:6210–6213. doi: 10.1002/anie.200501956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roots R, Holley W, Chatterjee A, Irizarry M, Kraft G. The formation of strand breaks in DNA after high-LET irradiation: A comparison of data from in vitro and cellular systems. Int J Radiat Biol. 1990;58:55–69. doi: 10.1080/09553009014551431. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikazono N, Pearson CG, O’Neill P, Thacker J. The roles of specific glycosylases in determining the mutagenic consequences of clustered DNA base damage. Nucl Acids Res. 2006;34:3722–3730. doi: 10.1093/nar/gkl503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalletta RA, Bernhard WA. Free radical yields in A:T polydeoxynucleotides, oligodeoxynucleotides, and monodeoxynucleotides at 4 K. Radiat Res. 1992;130:7–14. [PubMed] [Google Scholar]
- Swarts SG, Becker D, Sevilla M, Wheeler KT. Radiation-induced DNA damage as a function of hydration. II. Base damage from electron-loss centers. Radiat Res. 1996;145:304–314. [PubMed] [Google Scholar]
- Swarts SG, Gilbert DC, Sharma KK, Razskazovskiy Y, Purkayastha S, Naumenko KA, Bernhard WA. Mechanisms of direct radiation damage in DNA, based on a study of the yields of base damage, deoxyribose damage, and trapped radicals in d(GCACGCGTCG)2. Radiat Res. 2007;168:367–381. doi: 10.1667/RR1058.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Sonntag C. Free-radical-induced DNA damage. Springer-Verlag; Germany: 2006. [Google Scholar]
- Wallace SS, Yang N, Galick H. Attempted base excission repair of ionizing radiation damage in human lymphoblastoid cells produces lethal and mutagenic double strand breaks. DNA Repair. 2004;3:1323–1334. doi: 10.1016/j.dnarep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Wang W, Razskazovskii Y, Sevilla MD. Secondary radical attack on DNA nucleotides: reaction by addition to DNA bases and abstraction from sugars. Int J Radiat Biol. 1997;71:387–399. doi: 10.1080/095530097144003. [DOI] [PubMed] [Google Scholar]
- Wang W, Sevilla MD. Protonation of nucleobase anions in gamma-irradiated DNA and model systems. Which DNA base is the ultimate sink for the electron? Radiat Res. 1994;138:9–17. [PubMed] [Google Scholar]
- Ward JF. Some biochemical consequences of the spatial distribution of ionizing radiation-produced free radicals. Radiat Res. 1981;86:185–95. [PubMed] [Google Scholar]
- Weinfeld M, Rasouli-Nia A, Chaudhry MA, Britten RA. Response of base excision repair enzymes to complex DNA lesions. Radiat Res. 2001;156:584–589. doi: 10.1667/0033-7587(2001)156[0584:robere]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yan M, Becker D, Summerfield S, Renke P, Sevilla MD. Relative abundance and reactivity of primary ion radicals in g-irradiated DNA at low temperatures. 2. Single- vs double-stranded DNA. J Phys Chem. 1992;96:1983–9. [Google Scholar]
- Yokoya A, Cunniffe SMT, Stevens DL, O’Neill P. Effects of hydration on the induction of strand breaks, base lesions, and clustered damage in DNA films by α-radiation. J Phys Chem B. 2003;107:832–837. [Google Scholar]