Abstract
Hormetic dose response occurs for many endpoints associated with exposures of biological organisms to environmental stressors. Cell-based U- or inverted U-shaped responses may derive from common processes involved in activation of adaptive responses required to protect cells from stressful environments. These adaptive pathways extend the region of cellular homeostasis and are protective against ultimate cell, organ, and system toxicity. However, the activation of stress responses carries a significant energetic cost to the cell, leading to alterations of a variety of basal cellular functions in adapted or stressed cells. This tradeoff of resources between the unstressed and adapted states may lead to U-or inverted U-shaped dose response curves for some precursor endpoints. We examine this general hypothesis with chlorine, a prototype oxidative stressor, using a combination of cellular studies with gene expression analysis of response pathways and with computational modeling of activation of control networks. Discrete cellular states are expected as a function of exposure concentration and duration. These cellular states include normal functioning state, adaptive and stressed states at mild to intermediate exposures, and overt toxicity in the presence of an overwhelming concentration of stressors. These transitions can be used to refine default risk assessment practices that do not currently accommodate adaptive responses.
Keywords: Hormesis, adaptive response, homeostasis, oxidative stress, chlorine
BACKGROUND OF HORMESIS
The concept of hormesis encompasses a wide array of nonmonotonic biological responses that are either below or above control levels depending on the dose of the applied agent (Calabrese and Baldwin 2001a). Graphically, a hormetic dose response can be either a U- or inverted U-shaped curve, with the hormetic zone on average spanning a 10- to 20-fold dose range and the peak or nadir response 30–60% above or below control (Calabrese et al. 2007). The nonmonotonic biological response was first described in the late 19th century by Schulz who found that chemical fungicides such as mercuric chloride enhanced yeast metabolism at low doses but inhibited it at high doses (Schulz 1888). It wasn’t until the early 1940’s that the term hormesis was formally introduced by Southam and Ehrlich to define similar phenomena (Southam and Ehrlich 1943). Although by some, hormesis is thought to have a connection with homeopathy, a controversial therapeutic concept believing that extremely diluted toxicants are beneficial to human health, it is abundantly clear from accumulating evidence to date that hormesis is a real biological phenomenon (Calabrese and Baldwin 2001a; Calabrese and Baldwin 2001b). Hormetic responses have been observed at multiple levels of biological organizations with many physical/chemical stressors for a variety of biological endpoints. For example, U- or inverted U-shaped responses have been observed for DNA damage (Kitchin and Brown 1994), cellular fate such as proliferation, differentiation, and survival (Pi et al. 2008a), and for pathological endpoints such as carcinogenesis (Kitano et al. 1998). Despite a frequently observed phenomenon, hormetic dose response is used neither as the default nor as a secondary model for conducting chemical risk assessments. Application of these dose response relationships for risk assessment remains problematic because the biological basis of this phenomenon is not well-characterized.
Current efforts to explain nonmonotonic responses including hormesis suggest that these complex dose response curves may arise from a variety of mechanisms, depending on both the biological endpoints and type of inducing agents (Conolly and Lutz 2004). Further, the dose response for the various endpoints is also a function of time in the pathogenesis at which the observation occurs, e.g., according to standard toxicological testing sacrifice schedules. The underlying mechanisms for nonmonotonic responses may operate at different levels of the biological organizations involving interactions between multiple organs/tissues, cell types, or cellular components. Some biphasic responses occur in systems in which the input signal affects the endpoint through two parallel yet functionally opposing processes, each with a different sensitivity. A classic example of this is the biphasic response of blood vessels to adrenergic stimulants. At a low concentration, isoproterenol causes dilation of arteries by inhibiting smooth muscle contraction via β-receptors; whereas at a high concentration, it causes constriction of arteries by stimulating smooth muscle contraction via α-receptors (Fleisch et al. 1970). In the context of steroid hormone signaling, we recently proposed that homodimerization of steroid hormone receptors, an inherently nonlinear process, may be responsible for the nonmonotonic dose responses observed with certain steroid mimics including endocrine active chemicals and selective steroid receptor modulators (Li et al. 2007). In carcinogenesis, nonmonotonic relationships may arise from competing processes that have opposite effects on tumor formation but different dose dependencies (Andersen and Conolly 1998).
Despite the fact that an individual hormetic response may be tied to a specific cellular or physiological pathway(s) or processes(s), there have been several efforts made in the past to advance a generally unifying theory for hormesis. One particularly attractive hypothesis centers on the homeostatic adaptation of a biological system in response to perturbations. A fundamental feature of biological systems is robustness, i.e., the unusual ability to carry out basic functions nearly unaltered in spite of various perturbations imposed by changes in the internal or external environment (Kitano 2004). This robustness is maintained by an array of homeostatic control systems at both cellular and physiological levels, which are activated to compensate for perturbations, adapting the biological organisms to stressful environments. For example, DNA damage by radiation is an adaptive response in which a variety of DNA repair enzymes are activated to alleviate further damages by continued exposure to radiation. Calabrese and others argued that hormesis simply occurs as a result of overcompensation by the homeostatic control system exposed to stressors at low doses (Stebbing 1998; Calabrese 2001). While this is an appealing hypothesis, it remains unclear as to how overcompensation occurs mechanistically with an adaptive system. Here, we advance a hypothesis on the manner in which adaptive cellular responses may lead to hormesis.
ADAPTIVE ANTI-STRESS GENE REGULATORY NETWORK AND CELLULAR STATE TRANSITION
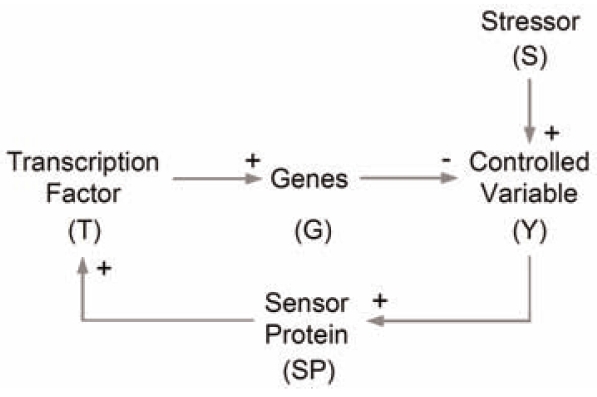
One aspect of robustness at the cellular level is the maintenance of a stable intracellular milieu in a constantly changing extracellular environment. In this context, environmental stressors usually disturb the concentrations of important molecular species in the cell that need to be kept in a tightly controlled range for normal cell function. From an engineering perspective, these molecular species are controlled variables, examples of which include reactive oxygen species (ROS), DNA adducts, glucose, and oxygen. To prevent these controlled variables from large and potentially harmful deviations from their basal operating concentrations, cells possess an array of anti-stress gene regulatory networks each responsible for handling a particular type of physical/chemical stress, such as oxidative stress, DNA damage, protein denaturation, and osmotic stress. These regulatory networks in cells are very complex, often involving multiple genes, proteins/enzymes, metabolic reactions, protein/protein and protein/DNA interactions, as exemplified by the antioxidant stress response (Kensler et al. 2006) and heat shock response (Kampinga 2006). Despite the vast complexity, most anti-stress gene regulatory networks can be conceptually viewed as a negative feedback circuit, which underlies the adaptive responses to many biological stressors (Fig. 1). An anti-stress gene regulatory network usually contains specialized protein molecules which can sense the level of controlled variables, which can be ROS, misfolded proteins, and DNA adducts, etc. External stressor-induced initial changes in the level of controlled variables are first detected by these molecular sensors. The signal is then relayed to activate specific transcription factors (in some cases the transcription factors themselves can serve directly as the sensor molecules). Closing the feedback loop, activated transcription factors upregulate expression of a suite of anti-stress genes, which encode metabolic enzymes working coordinately to counteract the changes in controlled variables brought about by the perturbing stressors. As a result, within a wide dose range of the stressors, the steady-state concentrations of controlled variables may not change as much due to the operation of this homeostatic control mechanism.
FIGURE 1.
Illustration of the adaptive negative feedback control scheme generalized for anti-stress gene regulatory networks responsible for maintaining cellular homeostasis.
To achieve a robust homeostatic control, i.e., to have a high resistance to perturbations in order to maintain controlled variables within a tightly regulated range, theoretical work has emphasized the importance of a high loop gain for a negative feedback-mediated control system. In this fashion, cells are able to take advantage of a myriad of gain-enhancing mechanisms in anti-stress gene regulatory networks to achieve robust homeostatic control (Zhang and Andersen 2007). These include multi-merization of transcription factors, anti-stress proteins, and enzymes, cooperative promoter binding, localized positive autoregulation of transcription factors or cofactors, and switch-like signaling such as the three-tiered MAPK cascade (Huang and Ferrell 1996). While it is essential to appreciate the role of a high loop gain in anti-stress responses, it is equally important to note that the control conferred by the feedback loop may operate at different capacities as the level of exposure to stressors varies. In a typical cellular homeostatic control system, the saturable nature of biochemical interactions and reactions dictates that the steady-state concentrations of the controlled variable undergo various phases as the dose of stressor increases (Zhang and Andersen 2007). Under relatively low-level exposure, the homeostatic control system operates responsively (i.e., the expression of anti-stress genes is upregulated markedly and metabolic enzymes are working at conditions far below saturation.), counteracting the perturbation to the controlled variable. This controlled phase is superlinear in appearance for the concentration of the controlled variable (Fig. 2A). With intermediate-level exposures, the feedback control system is less capable of maintaining homeostasis because anti-stress gene upregulation is approaching maximum induction. In this less controlled phase, the response curve of the controlled variable gradually changes into a sublinear response curve (in some cases, there could be a linear component toward the end), but the rise in the concentration of the controlled variable is still largely contained. Eventually, with sufficiently intense exposures, the rise of the controlled variable is markedly accelerated, entering a sublinear catastrophic phase. This final phase occurs because enzymatic reactions in the homeostatic control system responsible for keeping the controlled variable at safe levels in the cell are finally moving closer to saturation.
FIGURE 2.
Graphic illustration of the hypothesis that hormesis arises from the interplay between the adaptive response and enhanced energy expenditure required to operate the underlying homeostatic control system. (A, B) Typical steady-state dose response curves for the controlled variable (Y) and anti-stress gene expression (G), respectively, in an anti-stress gene regulatory network mediated via negative feedback (Zhang and Andersen 2007). The controlled variable Y transitions through controlled, less controlled, and catastrophic phases. (C, D) If the controlled variable and energy expenditure supporting anti-stress gene expression operate linearly but in opposite directions to affect a particular endpoint response, as described by R = C − αY + βG, then hormesis arises within the adaptive controlled region and part of the less controlled region because of the slow rise in Y and sharp rise in G. Shaded areas in C denote the differences between βG and αY with the sign of the difference indicated.
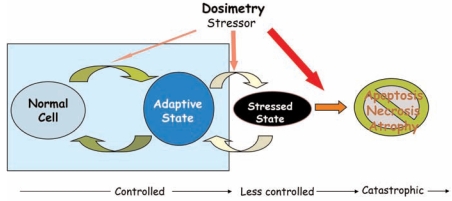
Maintaining controlled variables around their operating concentrations is crucial to normal cellular functions. Increasingly higher exposures to stressors, as illustrated in Fig 2A, are likely to be associated with transitions of cells through several distinct states of well-being (Fig. 3). Under exposure to low-dose stressors, very little change in controlled variable concentrations is expected due to strong homeostatic control. This controlled phase is associated with an adaptive state of the cells to stressors. In this state, cells may not appear much different from the unperturbed cells, but there are coordinate changes in gene expression to maintain cellular homeostasis. Further increase in the level of the stressor overwhelms the cellular capacity to handle the stressor. Under this condition, cells manage to survive, but with many cellular functions likely altered (e.g., cell cycle may be arrested) and innate defense systems activated (e.g., inflammatory response), thus cells enter a stressed state. Both the adaptive and stressed states are reversible such that when no longer exposed to the stressor, cells return to their normal state. At high stressor doses, the uncurbed rise in the controlled variable leads to cellular toxicity, which eventually kills the cells by initiating the apoptotic pathway or through necrosis.
FIGURE 3.
Illustrations of cellular state transition as the dose of exposure to stressors increases. Mild to moderate exposure shifts cells to an adaptive state because the homeostatic control is operating. Higher exposure moves cells to a stressed state because the limit of homeostatic control is reached. In this state the innate immune system may be activated leading to inflammatory responses. Cells in adaptive or stressed state can still return to normal, unstressed state after removal of the stressor. However, very high doses of stressors are likely to drive cells irreversibly to a toxic state, where apoptosis or necrosis occurs. Apoptosis is an active, programmed self-terminating process of the cell in the event that the cellular damage is too large to be repaired or worth repairing, or the cell’s continued survival is no longer benefiting the organism as a whole. The above three states are closely associated with the dose response transition for the controlled variable Y in Fig. 2A (i.e., controlled, less controlled, catastrophic phases).
HYPOTHESIS
Under conditions of mild to moderate stressor exposures, cells are at an adaptive state with slight deviations in the concentrations of controlled variables. This adaptation does not occur without a cost – the “behind-the-scene” upregulation of anti-stress genes involved in counteracting the perturbations (Fig. 2B) carries some significant energetic costs for the exposed cells. For example, in E coli, heat shock proteins, which help to refold denatured proteins to functionally-folded state, are upregulated to represent 20% of the total protein at 46°C vs. less than 2% at 30°C (Arsene et al. 2000). It is our hypothesis that this altered energy expenditure occurring in the adaptive cellular state, and possibly in the early state of the stressed state, may be responsible for hormetic changes in some cellular endpoints such as rate of proliferation, differentiation, or cell viability.
To illustrate our hypothesis, we can assume that the controlled variable (Y) and the energetic cost incurred from anti-stress gene expression (G) have opposite influences on a given endpoint response (R). To keep the model simple, we can further assume the relationship is linear with a coefficient of α and β, respectively (i.e., R = C − αY + βG, where C is a constant independent of Y and G). Since the adaptive (controlled phase) and some early part of the stressed (less controlled phase) state is characterized by a slow increase in Y but a sharp rise in G (Fig. 2A and 2B), the net effect on R will be positive under mild to moderate exposure conditions (Fig. 2C). At very high exposure conditions there is a failure of stress control. In this situation, the sharp rise in Y and flat change in G produce a negative net effect on the endpoint response (Fig. 2C). Consequently, over the whole dose range, the endpoint response would initially increase and then decrease, displaying a hormetic response profile (Fig. 2D).
AN EXAMPLE: OXIDATIVE STRESS
Living cells are constantly exposed to ROS including superoxide, hydrogen peroxide, and hydroxyl radicals. Endogenously, ROS are produced by the aerobic respiratory chain reactions in the mitochondria and by many biochemical reactions taking place in other organelles. Environmental exposure of cells to many chemicals, UV light, and ionizing radiations can increase ROS production, potentially disrupting cellular redox balance. Excessive ROS accumulation damages macromolecules including lipid, protein, and DNA, leading to membrane structural changes, protein malfunctions, and genomic instability. To control the impact of oxidative stressors, cells are equipped with a suite of antioxidant enzymes and small molecules to detoxify excess ROS and maintain intracellular ROS at appropriate levels. These antioxidant enzymes/molecules include superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutamate cysteine ligase (GCL), glutathione synthetase (GS), and reduced glutathione (GSH), etc.
As with many other control systems, redox homeostasis is maintained through negative feedback controls. In mammalian cells, cytosolic protein Keap1 (Kelch-like ECH-associated protein 1) is believed to be responsible for sensing the level of intracellular ROS (Motohashi and Yamamoto 2004). At basal conditions, Keap1 promotes ubiquitination and degradation of Nrf2 (nuclear factor erythroid 2-related factor 2), a transcription factor that binds to antioxidant response element (ARE). Nrf2 is thus kept at a low level. When cells are exposed to oxidative stressors, increased ROS oxidize several cysteine residues in Keap1. Oxidized Keap1 loses its ability to mediate Nrf2 ubiquitination, resulting in stabilization of Nrf2 (Kobayashi et al. 2006). As a consequence, Nrf2 accumulates via de novo synthesis then translocates to the nucleus. In addition, phosphorylation of Nrf2 by a variety of protein kinases is also believed to play certain roles in its activation (Yu et al. 2000; Bloom et al. 2002; Huang et al. 2002; Kang et al. 2002; Pi et al. 2007). Activated Nrf2 binds to AREs on the promoter regions of many antioxidant genes, upregulating their expression. The subsequently enhanced antioxidant capacity reduces cellular concentrations of ROS, restoring the redox equilibrium. An interdisciplinary group in our laboratory (The Hamner Institutes for Health Sciences) is beginning to use this oxidative stress gene regulatory network as a prototype homeostatic system to examine the hypothesis for hormesis noted in Fig. 2.
CHLORINE, OXIDATIVE STRESS, AND HORMESIS
While many chemicals can cause oxidative stress, our studies focused on chlorine as a prototype chemical. Chlorine is widely used in human society. It is a common water disinfectant, a synthetic intermediate for many commodity chemicals, and is used for textile and paper bleaching as well as in pharmaceuticals and cosmetics. Humans are exposed to chlorine in swimming pools, in household cleaners, and in severe cases, from accidental spills during its transportation (Evans 2005). Inhalation of chlorine gas can cause a range of respiratory disorders, including pulmonary edema, restrictive lung disease, and obstructive disease such as reactive airway dysfunction syndrome (Lehmler et al. 2005). When dissolved in an aqueous condition, such as the surface fluid in the respiratory tract, chlorine gas rapidly hydrolyzes to hypochlorous acid (HOCl) and hydrogen chloride (HCl). In solution, HOCl dissociates to form hypochlorite ion (OCl−). Sensory irritant responses in the nose were observed when exposed to less than 1 ppm of chlorine gas, whereas it takes many times more HCl to produce any irritant effects (Barrow et al. 1977). Thus it is believed that HOCl is the active form of irritant, and is also the active form of chlorine in bacteria-killing detergents. As a reactive oxidant, HOCl reacts with biological tissues, producing a variety of oxidized and chlorinated products including chlorinated aromatic amino acids such as chlorotyrosine. These products can serve as a local bio-marker for the exposure of HOCl in tissues.
The predominant mode of action for chlorine in the respiratory tract appears to be mediated through oxidative stress from HOCl, a strong ROS. Treatment of mouse macrophages with HOCl dose-dependently induced nuclear accumulation of Nrf2, the primary transcription factor mediating antioxidant stress response (Pi et al. 2008a). In addition, many Nrf2 downstream antioxidant genes were upregulated, including NADPH: quinone oxidoreductase 1, heme oxygenase-1, GCL, SOD, CAT, etc (Pi et al. 2008a). HOCl can penetrate the cell membrane to react directly with GSH (Winterbourn and Brennan 1997; Pullar et al. 1999). Therefore, the initial cellular response to HOCl included a decrease in intracellular GSH (Pi et al. 2008a). However, as GCL and GS, the two enzymes responsible for de novo GSH synthesis are upregulated through Nrf2 activation, decreases in the intracellular GSH level can be reversed, and eventually GSH may increase above control levels.
Plotting intracellular GSH levels vs. different HOCl concentrations shows a hormetic response at 12 hour after the onset of HOCl exposure (Fig. 4A). At HOCl concentrations less than 0.7 mM, GSH are above the basal level; however, further increases in HOCl concentration result in a decrease in GSH levels, and at 2.8 mM of HOCl, intracellular GSH decreases to below the basal level. This hormetic GSH response can be attributed to a similar hormetic dose response profile of GCLC, the catalytic subunit of GCL. The peak mRNA level of GCLC shows a maximum response at 0.7 mM of HOCl (Fig. 4B), and greater HOCl concentrations somehow suppress GCLC expression. A similar hormetic response in gene expression was also observed with other antioxidant genes such as NQO-1 (Fig. 4C). The repression of gene expression at high HOCl concentrations is not due to cytotoxicity as at the same concentration 90% of the cells are still viable, and other genes, not themselves involved in antioxidant response, are upregulated (data not shown). Importantly, cell viability, as measured by MTT assay, also displays a hormetic dose response. Exposure to low concentrations of HOCl causes up to 20% enhancement of cell viability whereas high concentrations reduce viability (Fig. 4D). When comparing the hormetic zones in all these responses (Fig. 4A – 4D), it appears that hormesis occurs in approximately the same dose range regardless of the endpoint. These nearly overlapping hormetic zones coincide with the adaptive cellular state, as the cell population seems to survive well in the dose range. The hormetic viability response was also observed in cells pre-treated with a moderate concentration of oxidants. As indicated in Fig. 4D, a previous exposure to a low concentration of HOCl or tert-butylhydroquinone shifts the dose response curve to the right for subsequent HOCl treatment, while preserving the nonmonotonic nature. It needs to be noted that the hormetic change in cell viability observed with HOCl appears to represent a “conserved” response profile associated with exposure to oxidative stressors. For instance, arsenic, an oxidative stressor that can activate Nrf2-mediated adaptive response (Pi et al. 2003), also increases cell viability at low doses and decreases it at high doses (Pi et al. 2008b). A similar effect was also observed with hydrogen peroxide in yeasts (Davies et al. 1995).
FIGURE 4.
Hormetic responses in RAW 264.7 mouse macrophages treated with HOCl. (A) Intracellular GSH levels at 12 h after HOCl treatment. (B, C) Gene expression of GCLC and NQO-1, respectively, at 6 h after HOCl treatment. (D) Cell viability at 24 h (measured with MTT assay) after HOCl treatment in the absence of any pretreatment (dashed line) or in the presence of previous exposure to 0.7 mM HOCl (dotted line) or 5 μM tert-butylhydroquinone (solid line). Part of the data are adapted from (Pi et al. 2008a). * indicates P<0.05 compared with controls.
Nel has hypothesized that under exposure to increasing concentrations of oxidative stressors, there is a hierarchical activation of different types of cellular pathways/responses (Xiao et al. 2003). With no or extremely low-level oxidative exposures, cells maintain their normal functions without any significant alterations. A mild or moderate oxidative exposure will activate the Nrf2-mediated antioxidant response by inducing phase II and antioxidant enzymes, which are responsible for keeping ROS at relatively low levels. This tier I response is adaptive in nature, and cells can survive without markedly altered functions. A further increase in the stressor dose starts to overwhelm the Nrf2-mediated antioxidant control system. As a result, oxidative stressors move the cell down the response hierarchy, activating tier II NF-kB-mediated inflammatory response. Additional increase in the oxidative stressor dose will drive cells into tier III response, which activates apoptotic pathways leading to cell death. Our findings with macrophages treated with HOCl are consistent with this sequence of responses. HOCl at concentrations less than 0.7 mM activates antioxidant gene expression. At higher concentrations antioxidant gene expression is repressed while inflammatory gene markers such as IL-6 and IL-1 β are activated (Woods et al. 2008). At even greater HOCl doses, reduced cell viability is observed (Fig. 4D), indicating cells may be entering tier III cytotoxic phase. The hormetic change in cell viability observed at relatively low HOCl concentrations coincides with tier I antioxidant response, suggesting that hormesis is closely coupled to the activation of adaptive homeostatic mechanisms.
CONCLUSIONS
The phenomena of hormesis and toxicity thresholds are likely related to activation of adaptive pathways responsible for cellular and physiological homeostasis. Before hormesis can be used on a large scale in risk assessment, several prototype chemicals and adaptive response models exemplifying hormetic responses need to be well-characterized to understand the underlying homeostatic responses. These prototypes may include irritant gas such as chlorine, discussed here, heavy metals, as well as receptor-mediated responses from hepatic enzyme inducers or other transcriptionally active xenobiotics. A chemical is likely to impinge upon more than one toxicity pathway, which is interconnected into responsive networks within the cell, as suggested by the tiered responses observed with HOCl. Therefore, the overall cellular responses to a particular perturbation are governed by the systems-level behaviors of the networks. Characterization of the underlying mechanisms of hormetic responses is an interdisciplinary effort requiring integration of dosimetry, in vitro and in vivo measurement of various endpoints, functional genomic mapping of the underlying biochemical pathways, and lastly, computational formulation of the adaptive pathways and networks that can test dose response hypotheses quantitatively. In the absence of well-developed examples revealing the systems-level mechanistic basis for hormesis and nonmonotonic responses, low-dose extrapolations employed in risk assessments will have to stay wedded to the low-dose linear and threshold linear methodologies that are now favored. The time has come to move from commenting on the frequency of observing hormesis to a commitment to understanding the biological mechanisms that control these frequently hormetic dose response relationships.
Footnotes
CONFLICT OF INTERESTS AND DISCLAIMER
The authors have declared that there is no conflict of interests. This document has been subjected to review by the National Health and Environmental Effects Research Laboratory of the U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The U.S. Government has the right to retain a nonexclusive, royalty-free copyright covering this article.
REFERENCES
- Andersen ME, Conolly RB. Mechanistic modeling of rodent liver tumor promotion at low levels of exposure: An example related to dose-response relationships for 2,3,7,8-tetra-chlorodibenzo-p-dioxin. Hum Exp Toxicol. 1998;17:683–90. 701–4, 708–18. doi: 10.1177/096032719801701208. [DOI] [PubMed] [Google Scholar]
- Arsene F, Tomoyasu T, Bukau B. The heat shock response of escherichia coli. Int J Food Microbiol. 2000;55:3–9. doi: 10.1016/s0168-1605(00)00206-3. [DOI] [PubMed] [Google Scholar]
- Barrow CS, Alarie Y, Warrick JC, Stock MF. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch Environ Health. 1977;32:68–76. doi: 10.1080/00039896.1977.10667258. [DOI] [PubMed] [Google Scholar]
- Bloom D, Dhakshinamoorthy S, Jaiswal AK. Site-directed mutagenesis of cysteine to serine in the DNA binding region of nrf2 decreases its capacity to upregulate antioxidant response element-mediated expression and antioxidant induction of nad(p)h:Quinone oxidoreductase1 gene. Oncogene. 2002;21:2191–200. doi: 10.1038/sj.onc.1205288. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Overcompensation stimulation: A mechanism for hormetic effects. Crit Rev Toxicol. 2001;31:425–70. doi: 10.1080/20014091111749. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. The frequency of u-shaped dose responses in the toxicological literature. Toxicol Sci. 2001a;62:330–8. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: A generalizable and unifying hypothesis. Crit Rev Toxicol. 2001b;31:353–424. doi: 10.1080/20014091111730. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007 doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Conolly RB, Lutz WK. Nonmonotonic dose-response relationships: Mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol Sci. 2004;77:151–7. doi: 10.1093/toxsci/kfh007. [DOI] [PubMed] [Google Scholar]
- Davies JM, Lowry CV, Davies KJ. Transient adaptation to oxidative stress in yeast. Arch Biochem Biophys. 1995;317:1–6. doi: 10.1006/abbi.1995.1128. [DOI] [PubMed] [Google Scholar]
- Evans RB. Chlorine: State of the art. Lung. 2005;183:151–67. doi: 10.1007/s00408-004-2530-3. [DOI] [PubMed] [Google Scholar]
- Fleisch JH, Maling HM, Brodie BB. Beta-receptor activity in aorta; variations with age and species. Circ Res. 1970;26:151–62. doi: 10.1161/01.res.26.2.151. [DOI] [PubMed] [Google Scholar]
- Huang CY, Ferrell JE., Jr Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A. 1996;93:10078–83. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HC, Nguyen T, Pickett CB. Phosphorylation of nrf2 at ser-40 by protein kinase c regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–74. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- Kampinga HH. Chaperones in preventing protein denaturation in living cells and protecting against cellular stress. Handb Exp Pharmacol. 2006:1–42. doi: 10.1007/3-540-29717-0_1. [DOI] [PubMed] [Google Scholar]
- Kang KW, Choi SH, Kim SG. Peroxynitrite activates nf-e2-related factor 2/antioxidant response element through the pathway of phosphatidylinositol 3-kinase: The role of nitric oxide synthase in rat glutathione s-transferase a2 induction. Nitric Oxide. 2002;7:244–53. doi: 10.1016/s1089-8603(02)00117-9. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the keap1-nrf2-are pathway. Annu Rev Pharmacol Toxicol. 2006 doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kitano H. Biological robustness. Nat Rev Genet. 2004;5:826–37. doi: 10.1038/nrg1471. [DOI] [PubMed] [Google Scholar]
- Kitano M, Ichihara T, et al. Presence of a threshold for promoting effects of phenobarbital on diethylnitrosamine-induced hepatic foci in the rat. Carcinogenesis. 1998;19:1475–80. doi: 10.1093/carcin/19.8.1475. [DOI] [PubMed] [Google Scholar]
- Kitchin KT, Brown JL. Dose-response relationship for rat liver DNA damage caused by 49 rodent carcinogens. Toxicology. 1994;88:31–49. doi: 10.1016/0300-483x(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate nrf2 through inhibition of ubiquitination activity of keap1. Mol Cell Biol. 2006;26:221–9. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler HJ, Robertson LW, Garrison AW, Kodavanti PR. Effects of pcb 84 enantiomers on [3h]-phorbol ester binding in rat cerebellar granule cells and 45ca2+-uptake in rat cerebellum. Toxicol Lett. 2005;156:391–400. doi: 10.1016/j.toxlet.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Li L, Andersen ME, Heber S, Zhang Q. Non-monotonic dose-response relationship in steroid hormone receptor-mediated gene expression. J Mol Endocrinol. 2007;38:569–85. doi: 10.1677/JME-07-0003. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP. Transcription factor nrf2 activation by inorganic arsenic in cultured keratinocytes: Involvement of hydrogen peroxide. Exp Cell Res. 2003;290:234–45. doi: 10.1016/s0014-4827(03)00341-0. [DOI] [PubMed] [Google Scholar]
- Pi J, Bai Y, et al. Molecular mechanism of human nrf2 activation and degradation: Role of sequential phosphorylation by protein kinase ck2. Free Radic Biol Med. 2007;42:1797–806. doi: 10.1016/j.freeradbiomed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Zhang Q, Woods CG, Wong V, Collins S, Andersen ME. Activation of nrf2-mediated oxidative stress response in macrophages by hypochlorous acid. Toxicol Appl Pharmacol. 2008a;226(3):236–43. doi: 10.1016/j.taap.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Pi J, Diwan BA, Sun Y, Qu W, He Y, Styblo M, Liu J, Waalkes MP. Arsenic-induced malignant transformation of human keratinocytes: Involvement of nrf2. 2008b doi: 10.1016/j.freeradbiomed.2008.05.020. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullar JM, Winterbourn CC, Vissers MC. Loss of gsh and thiol enzymes in endothelial cells exposed to sublethal concentrations of hypochlorous acid. Am J Physiol. 1999;277:H1505–12. doi: 10.1152/ajpheart.1999.277.4.H1505. [DOI] [PubMed] [Google Scholar]
- Schulz H. Uber hefegifte. Pflugers Archiv fur die gesamte Physiologie des Menschen und der Tiere. 1888;42:517–41. [Google Scholar]
- Southam CM, Ehrlich J. Effects of extracts of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–24. [Google Scholar]
- Stebbing AR. A theory for growth hormesis. Mutat Res. 1998;403:249–58. doi: 10.1016/s0027-5107(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Brennan SO. Characterization of the oxidation products of the reaction between reduced glutathione and hypochlorous acid. Biochem J. 1997;326 (Pt 1):87–92. doi: 10.1042/bj3260087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods CG, Zhang Q, Thomas RS, Boellmann F, Wang J, Wolf RC, Andersen ME, Pi J. Regulatory mechanism of nrf2 activation by hypochlorous acid and concomitant activation of cellular inflammatory pathways. Society of Toxicology Annual Meeting; Seattle, Washington. 2008. Abstract #2026. [Google Scholar]
- Xiao GG, Wang M, Li N, Loo JA, Nel AE. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003;278:50781–90. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- Yu R, Chen C, Mo YY, Hebbar V, Owuor ED, Tan TH, Kong AN. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a nrf2-dependent mechanism. J Biol Chem. 2000;275:39907–13. doi: 10.1074/jbc.M004037200. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Andersen ME. Dose response relationship in anti-stress gene regulatory networks. PLoS Comput Biol. 2007;3:e24. doi: 10.1371/journal.pcbi.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]