Abstract
The focus of this report is on the development of an improved DNA immunization protocol, which takes advantage of the strengths of DNA immunization, as well as those associated with adjuvant delivered by transcutaneous immunostimulatory (IS) patches. Because transcutaneous delivery of adjuvants to the skin at the vaccination site has been shown to amplify the immune response to protein antigens, we hypothesized that the same IS patch when placed on the skin at the site of DNA injection could further enhance the immune response to a DNA influenza vaccine. We have combined an influenza DNA vaccine, hemagglutinin fused with three copies of complement C3d, to enhance uptake and antigen presentation, with an IS patch containing heat-labile enterotoxin from Escherichia coli. Coadministration of a potent adjuvant in IS patches placed on the skin at the site of DNA vaccination dramatically amplifies anti-influenza antibody immune response. Supplementing DNA vaccines with IS patches may be a particularly valuable strategy because DNA vaccines can be rapidly modified in response to mutations in pathogens, and individuals with compromised immune systems such as transplant patients and the elderly will benefit from the enhanced antibody response induced by the IS patches.
Introduction
Vaccine strategies, such as influenza virus vaccination of the elderly, are highly effective in preventing disease but provide protection for only those patients who mount effective humoral response to the target antigen (Guebre-Xabier et al., 2003). Moreover, because the elderly commonly suffer to some extent from immunosenescence, generating adequate levels of virus-neutralizing antibodies with a vaccine presents a real challenge (Haynes and Swain, 2006). Adjuvants improve the magnitude and rates of immune responses, but their potency must be attenuated to minimize adverse side effects associated with them. Transcutaneous delivery of strong adjuvants such as heat-labile enterotoxin from Escherichia coli (LT) induces potent immune responses due to the LT-induced migration of activated antigen-presenting cells (APCs) from the skin to the proximal draining lymph node (Glenn et al., 2003). We have previously shown that LT delivered alone in an immunostimulating (LT-IS) patch placed on the skin at the site of vaccine injection can significantly amplify the immune response to injected vaccines with minimal adverse reactions at the site of immunization. Similarly, influenza virus–specific T cells isolated from the lungs show increased levels of gamma interferon and interleukin-4 (IL-4) production (Guebre-Xabier et al., 2003). An LT-IS patch placed near an injected vaccine also leads to increased levels of hemagglutination inhibition titers, enhanced mucosal immunoglobulin A responses, and enhanced antigen presentation (Guebre-Xabier et al., 2004). However, due to significant problems associated with producing contemporary influenza virus vaccines, such as the cost of producing them and the long period of time required to produce sufficient quantities of a new version of the vaccine, the ability to respond to new mutant pathological strains of the virus is a critical area of concern.
DNA vaccines represent a viable alternative to classical vaccines, which rely on attenuated viruses or subunit vaccines (Kieber-Emmons et al., 2000; Laddy and Weiner, 2006; Wheeler et al., 2006). DNA vaccines have several advantages compared to traditional vaccines: they are more stable, less expensive, easy to modify in response to viral mutations, and safer than subunit or viral-based vaccines. In addition, DNA vaccines can be modified so that genes encoding the desired antigen(s) can be targeted to specific cellular localizations, thereby promoting the desired type of immune response (Chattergoon et al., 1997; Henke, 2002; Peachman et al., 2003). The immune response to DNA immunization can also be enhanced by using molecular adjuvant(s) (immune modulators), such as cytokines in conjunction with the immunogen, which can direct the T helper cell toward the desired pathway (Laddy and Weiner, 2006). Another example of a molecular adjuvant is the C3d fragment of complement C3, which was previously shown to enhance antibody formation following immunization with recombinant hen egg lysozyme, a model antigen, containing three tandem repeats of C3d (3C3d) (Dempsey et al., 1996). More recently, we have used 3C3d as a molecular adjuvant to induce more rapid production of high-affinity protective anti-influenza hemagglutinin (HA) antibodies after DNA immunization of mice (Ross et al., 2000; Mitchell et al., 2003; Watanabe et al., 2003). However, the immune response induced by DNA vaccines is generally lower and requires a significantly longer period of time to reach maximal antibody titers in large animals and humans than that induced by classical vaccines (MacGregor et al., 1998; van Rooij et al., 1998). Therefore, additional improvements in DNA vaccine design are necessary before this approach can be widely utilized for successful DNA vaccination in humans.
The focus of this report is on the development of an improved DNA immunization protocol, which takes advantage of the strengths of DNA immunization, as well as those associated with transcutaneous LT-IS patches. Because the augmentation of immune response by transcutaneous delivery of adjuvants to the skin at the vaccination site is safer than when the adjuvant is injected with the vaccine (Glenn et al., 2003), and because we have previously demonstrated that LT-IS patch significantly improves antibody responses to influenza vaccination in the elderly (Frech et al., 2005), we hypothesized that the same LT-IS patch when placed on the skin at the site of DNA injection could augment the immune responses to our prototype DNA influenza vaccine. In this report we demonstrate for the first time that application of an LT-IS patch significantly enhances the onset and amplitude of the antibody response, as well as the T cell response, to gene gun immunization with the plasmid encoding secreted hemag-glutinin (HA) fused with 3 copies of complement component C3d (psHA-3C3d) vaccine compared to the immune response induced by the DNA immunogen alone.
Materials and Methods
Animals
Eight- or 10-week-old female BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were housed in a temperature-controlled and light-cycle-controlled facility, and their care was according to the guidelines of the National Institutes of Health and the University of California, Irvine. All experiments involving mice were performed at the University of California, Irvine, under an approved Institutional Animal Care and Use Committee protocol, which was issued to the principal investigator and the corresponding author (D.H.C.). The manuscript does not contain human studies.
DNA vaccine and immunizations
DNA plasmids expressing a secreted form of influenza HA (A/PR/8/34, H1H1) fused with three copies of murine C3d component were previously described (Ross et al., 2000). Immunization of mice with plasmids (two independent experiments with six animals for each experimental or control group) was performed on shaved abdominal skin using the Helios gene gun (Bio-Rad, Hercules, CA) as we described (Ghochikyan et al., 2003). Briefly, immediately prior to immunization, mice were anesthetized by intraperitoneal injection of nembutal solution. Mice were shaved and bombarded with two shots of 1 µg of DNA per 0.5 mg of gold beads (Bio-Rad) at a helium pressure setting of 400 psi. The skin was pretreated by hydration with saline-soaked gauze, and the stratum corneum was disrupted by buffing with fine-grade emery paper strips. Immediately following the immunization (0 h) or after 24 or 48 h of DNA bombardment, LT-IS patches (IOMAI, Gaithersburg, MD) were applied to hydrated skin of experimental mice for approximately 18 h. Control mice were immunized with the same DNA vaccine alone or in combination with placebo patches, which lacked the LT. Another control group did not receive the DNA vaccine, but LT-IS patches were applied to the skin as described above for LT-IS patches. Mice were immunized three more times by the same method biweekly, and sera were collected 7–8 days after each immunization.
Enzyme-linked immunosorbent assay for detection of anti-HA antibodies
Anti-influenza (or anti-HA) antibodies were measured in plasma of immunized and control mice as we described previously (Robinson et al., 1997). Briefly, the wells of Immulon II 96-well plates (Dynex Technologies, Chantilly, VA) were coated with killed influenza virus (pH 9.7, 2 h, 37°C) followed by three washes. Plates were blocked with 3% dry milk in Tris buffered saline containing 0.05% Tween-20 (TTBS). Different dilutions of sera from experimental and control mice were added into the wells to detect the endpoint antibody titers for these immunized groups. After incubation and washing, horseradish peroxidase (HRP)–conjugated anti-mouse immunoglobulin G (IgG) was added as recommended by the manufacturer (Jackson ImmunoResearch, West Grove, PA). Plates were incubated and washed, and the 3,3′,5,5′-tetramethylbenzidine peroxidase substrate (Pierce Biotechnology, Rockford, IL) was added to develop the reaction. The reaction was stopped by adding 2M sulfuric acid. All the plates were analyzed spectrophotometrically at 450 nm. To determine the specific isotypes present, sera were pooled, and tested in duplicate. For detection of mouse IgG1, IgG2a, IgG2b, or IgM isotypes, we used anti-mouse Ig-subclass-specific HRP-conjugated secondary antibodies (Zymed, San Francisco, CA).
T cell proliferation
Analyses of T cell proliferation were performed in splenocyte cultures from individual animals as we previously described (Cribbs et al., 2003). However, to decrease non-specific activation of splenocytes, we used HL-1 serum-free synthetic medium (Cambrex, Baltimore, MD) without fetal bovine serum. Splenocytes (5 × 105 in 100 µL) from experimental and control mice were restimulated in vitro with purified HA protein (provided by Dr. T. Ross) at a concentration 10 µg/mL. Cells were first incubated for 72 h, and then 1 µCi 3[H]thymidine (Amersham Pharmacia Biotech, Pistcataway, NJ) was added to each well for 16–18 h. Cells were harvested using a Tomtec Mach III (Tomtec, Hamden, CT) harvester, and 3[H]thymidine uptake (cpm) was counted on a MicroBeta 1450 Trilux scintillation counter (Wallac Oy, Turku, Finland). The stimulation index was calculated as previously described (Cribbs et al., 2003).
Statistical analysis
Statistical parameters (average value and standard deviation) were calculated using GraphPad Prism 3.03 software. Statistical differences between groups were analyzed by one-way analysis of variance (ANOVA) and Tukey’s multiple comparison posttest (GraphPad Prism 3.03 software). A p<0.05 was considered statistically different.
Results
Effect of LT-IS patch on the humoral immune response to DNA immunization
We hypothesized that stronger immune responses could be elicited by a combination of molecular and conventional adjuvants, especially when the adjuvants utilized different mechanisms for amplifying the response to the vaccine. To test this hypothesis, we examined the role of adjuvant patches in enhancing the anti-HA antibody responses in BALB/c mice vaccinated with psHA-3C3d. Differences in the anti-influenza titers produced in response to psHA-3C3d immunogen were analyzed in sera from immunized animals after each boost. Anti-HA antibody titers in the sera of mice immunized with psHA-3C3d followed by patch application were significantly higher than in the sera of mice immunized with psHA-3C3d only, or in combination with placebo patches (p<0.001) at each time point (Fig. 1A). Importantly, after only two injections with DNA plasmid plus LT-IS patches, there were detectable levels of specific anti-HA antibodies, which were further enhanced after the third and fourth injections. Thus, IS patches helped prime and amplify the antibody response to the DNA vaccine.
FIG. 1.
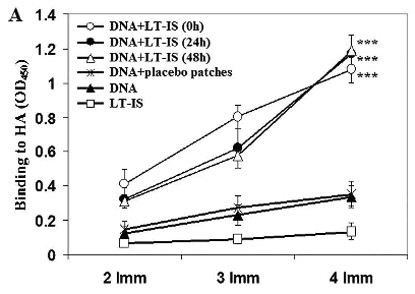
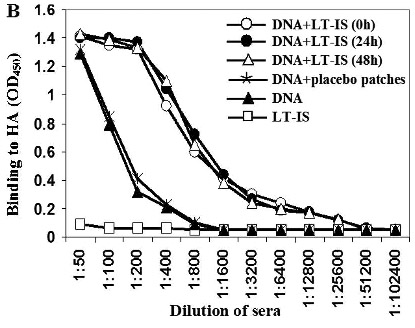
(A) Anti-influenza antibody responses enhanced by combining DNA vaccination with LT-IS patches. Application of LT-IS patches following DNA vaccination (at 0, 24, and 48 h) induces significantly higher amount of anti-influenza antibodies compared to controls. Binding to viral antigens was analyzed in sera of mice at dilution 1:250, and average values from one out of two independent enzyme-linked immunosorbent assay experiments are presented (similar results were obtained in the second experiment). Error bars represent SD; ***p<0.001. (B) Adjuvant (LT) administrated as an immunostimulant (IS) patch increases titers of antiviral antibodies in mice vaccinated with DNA immunogen. Results from one out of two independent experiments are presented (similar results were obtained in the second experiment).
Temporal relationship between LT-IS patch application and the humoral immune response to DNA immunization
We investigated whether the time of LT-IS patch application was critical to the humoral immune response to DNA immunization because the time course of protein expression induced by DNA immunization, and the antigen uptake and presentation by APCs are not well defined. Therefore, we decided to apply the LT-IS patches at different time points postimmunization. Interestingly, we did not observe statistically significant differences in the titers of antibody elicited in the three experimental groups of mice immunized with DNA plasmid followed by application of LT-IS patches at 0, 24, or 48 h post-DNA immunization (Fig. 1A). Serial dilutions of sera collected from DNA-immunized and control mice were used to determine the endpoint titers of anti-influenza anti-bodies after the final immunization (Fig. 1B). Experimental mice that received four doses of the psHA-3C3d DNA vaccine accompanied by LT-IS patches induced the highest titers (>1:12,800) of anti-HA antibodies. This titer was approximately 30 times greater than that generated in mice immunized with DNA vaccine only, or psHA-3C3d combined with placebo patch lacking the LT adjuvant (Fig. 1B).
Effect of LT-IS patches on antibody isotype in response to DNA immunization
Antibody isotyping has been used as an indirect measure of the contribution of Th1 (IgG2a) and Th2 (IgG1) cytokines during the stimulation of the humoral response (Finkelman et al., 1990). Thus, we measured production of IgG1, IgG2a, IgG2b, and IgM anti-influenza antibodies in the sera of immune BALB/c mice. Previously, it was shown that a model antigen fused with 3C3d induced predominantly IgG1 antibodies in CBA mice immunized intramuscularly (Dempsey et al., 1996). More recently, we have demonstrated that splenocytes of another strain of mice (BALB/c) immunized with psHA-3C3d also produced primarily Th2-type cytokine, IL-4 (Guebre-Xabier et al., 2003). Thus, as expected, our control group of BALB/c mice injected with psHA-3C3d produced influenza-specific antibodies of IgG1 isotype (Fig. 2). Mice immunized with psHA-3C3d and supplemented with the LT-IS patch immediately, or after 24 and 48 h following DNA immunization also induce anti-influenza antibodies of primarily of the IgG1 isotype (Fig. 2). The calculated IgG1/IgG2a ratio following immunization with DNA immunogen with or without LT-IS indicates that the both immunization protocols induce a highly polarized Th2-mediated immune response. Thus, in our experiments the LT-IS patches do not change the isotype profile of antibodies generated in BALB/c mice vaccinated with psHA-3C3d (Fig. 2).
FIG. 2.
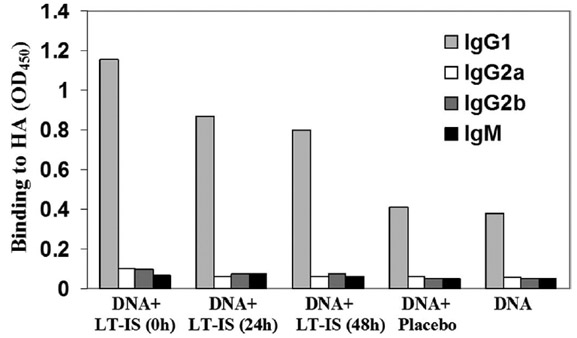
Detection of IgG1, IgG2a, IgG2b, and IgM subclasses of anti-influenza antibodies in pooled sera (dilution 1:250) collected from individual mice after the last immunization. Immunization with DNA vaccine alone or in combination with placebo patch induced production of antiviral antibodies of IgG1 isotype. Application of LT-IS patches following DNA vaccination does not change the isotype profile of anti-influenza antibodies. Results from one out of two independent experiments are presented (similar results were obtained in the second experiment).
Effect of LT-IS patches on the T cell response to DNA immunization
T cell help is an essential factor for robust B cell activation, and since LT-IS patches enhanced antibody response, we examined whether the patches also amplified the anti-HA-induced T cell proliferation following DNA vaccination. Ten days postimmunization, splenocytes from mice were isolated and restimulated in vitro with purified HA protein. As expected, splenocytes from mice immunized with psHA-3C3d plus LT-IS patches induce significantly stronger T cell proliferation responses to the viral antigen than those from mice immunized with psHA-3C3d alone (Fig. 3). As expected, we saw no difference in HA-induced T cell proliferation when LT-IS patches were applied at the different time points (Fig. 3).
FIG. 3.
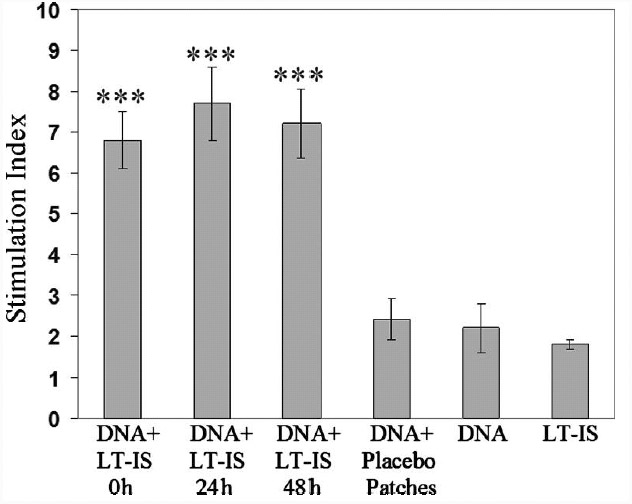
T cell proliferation in mice immunized with DNA vaccine combined with LT-IS patch. Splenocytes from immunized and control mice were restimulated in vitro with purified HA antigen. Only application of LT-IS patches following DNA vaccination induces significant proliferation of antigen-specific T cells. LT-IS patch, DNA vaccine alone, or combination of DNA vaccine and placebo patch induced very low proliferation of splenocytes. Pools of splenocytes from each group were analyzed, and two experiments were performed with 12 mice in each group. Mean values of stimulation index from two independent experiments are presented. Error bars represent SD; ***p<0.001.
Mechanisms involved in the LT-IS patch–induced amplification of the immune response to gene gun–mediated DNA immunization
We have demonstrated that the transcutaneous delivery of the LT adjuvant via IS patches can significantly enhance the B and T cell response to gene gun–mediated DNA immunization, without altering the Th phenotype. Previously, Dempsey et al. (1996) suggested three possible mechanisms for enhancement of immune responses by C3d fusion proteins. All these mechanisms are based on binding of C3d to CD21/CR2 receptor expressed on B cells, as well as on professional APCs. CD21/CR2 is coexpressed as a noncovalent complex with CD19, which functions as a specialized membrane adaptor protein for antigen-specific B cell receptors (BCRs) (Ahearn et al., 1996). BCR activation induces the phosphorylation of CD19, which results in the activation of lipid and protein kinases and subsequent increases in Ca2+ influx (O’Rourke et al., 1998; Brooks et al., 2000). It has been demonstrated previously that following antigen binding, the BCR moves into cholesterol/sphingolipid-rich membrane lipid raft microdomains (Cheng et al., 1999). Translocation of both CD19/CD21 complex and BCR into lipid rafts was shown to occur after binding of cells to an antigen tagged with C3d. Importantly, CD19/CD21 complex significantly prolongs BCR residency in lipid rafts and also stimulates signaling through this antigenic receptor (Cherukuri et al., 2001). Thus, it is likely that secreted HA molecule fused with 3 copies of complement component C3d (sHA-3C3d) molecule synthesized in vivo after DNA immunization has the capacity to bind both antigen-specific BCR and CD21/CD19 and act as a molecular adjuvant for amplifying the humoral response to HA (Ross et al., 2000). In addition, we have previously shown that topical delivery of adjuvant to the skin significantly augments protective humoral and cellular immune responses to influenza virus antigens likely through activation of skin dendritic cells (Guebre-Xabier et al., 2003).
Another desirable feature of vaccines is to provide a strong memory response so that subsequent boosts induced a therapeutic level of protective antibodies. Because the CD21 molecules expressed on professional APCs also promote the development and maintenance of memory B cells (Dempsey et al., 1996), we suggest that combining the LT-IS patches with 3C3d molecular adjuvant also will induce enhanced memory responses following DNA vaccination (Guebre-Xabier et al., 2003). The studies presented here establish that both humoral and cellular immune responses induced by a DNA vaccine can be further enhanced by topical delivery of LT-IS adjuvant directly to the skin at the site of DNA immunization.
Discussion
Vaccines can be highly effective in preventing diseases caused by infectious agents; however, inducing an adequate immune response without significant adverse events represents a significant challenge to vaccine development (Guebre-Xabier et al., 2003; Haynes and Swain, 2006). We believe that a combinatorial approach to DNA vaccine design that includes a molecular adjuvant in the DNA immunogen, which is further supplemented with IS patches, can be widely utilized for successful DNA vaccination in humans. Desirable features of this approach include the transcutaneous delivery of potent adjuvants, which can safely promote potent immune responses to vaccines (Glenn et al., 2003), and secondly the use of DNA vaccine technology that can be rapidly modified in response to mutations in pathogens and configured to include a molecular adjuvant that targets antigen uptake by APCs (Dempsey et al., 1996; Kieber-Emmons et al., 2000; Laddy and Weiner, 2006; Wheeler et al., 2006).
In this report we demonstrate that application of LT-IS patch significantly enhances the onset and amplitude of the antibody response, as well as the T cell response, to gene gun immunization with the psHA-3C3d vaccine compared to the immune response induced by the DNA immunogen alone. In addition, we found that the time of LT-IS patch application was not critical to the humoral immune response to DNA immunization because there were no significant differences in the titers of antibody elicited when the LT-IS patches were applied at the same time as the DNA immunization, or at 24 or 48 h postimmunization (Fig. 1A). This result is most likely due to the continuous expression of the psHA-3C3d in the skin, which provides a chronic source of antigen that can be processed by APCs recruited by application of the LT-IS patches. Interestingly, in our experiments the LT-IS patches did not change the isotype profile of antibodies generated in BALB/c mice vaccinated with psHA-3C3d (Fig. 2). However, because BALB/c mice are prone to a Th2-biased immune response (Mills et al., 2000; Wang et al., 2003), future studies using different strains of mice will be necessary to demonstrate that the LT adjuvant patches do not convert the psHA-3C3d–induced immune response to a Th1 phenotype. Conversely, if the LT adjuvant were to induce a switch from a Th2-preferred immune response in other strains of mice, then substitution of LT with cholera toxin in the IS patches may be desirable (Glenn et al., 1998; Eriksson et al., 2003; Lavelle et al., 2004; Su et al., 2004; Nikolic et al., 2007). Based on the results presented in this report, we propose that the combination of DNA immunization with IS patches should reduce the number of injections necessary for elicitation of potent immune responses in humans, which provides support for a new strategy for safely enhancing the immune response to DNA vaccination.
Acknowledgments
Dr. Nina Movsesyan was supported by NIA training Grant AG00096. This work was supported by RO1 Grants NIA AG-2041 and NINDS NS-50895 from the National Institutes of Health to Dr. David H. Cribbs.
References
- Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, Carroll MC. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- Brooks SR, Li X, Volanakis EJ, Carter RH. Systematic analysis of the role of CD19 cytoplasmic tyrosines in enhancement of activation in Daudi human B cells: clustering of phospholipase C and Vav and of Grb2 and Sos with different CD19 tyrosines. J Immunol. 2000;164:3123–3131. doi: 10.4049/jimmunol.164.6.3123. [DOI] [PubMed] [Google Scholar]
- Chattergoon M, Boyer J, Weiner DB. Genetic immunization: a new era in vaccines and immune therapeutics. FASEB J. 1997;11:753–763. doi: 10.1096/fasebj.11.10.9271360. [DOI] [PubMed] [Google Scholar]
- Cheng PC, Dykstra ML, Mitchell RN, Pierce SK. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J Exp Med. 1999;190:1549–1560. doi: 10.1084/jem.190.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherukuri A, Cheng PC, Sohn HW, Pierce SK. The CD19/CD21 complex functions to prolong B cell antigen receptor signaling from lipid rafts. Immunity. 2001;14:169–179. doi: 10.1016/s1074-7613(01)00098-x. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- Eriksson K, Fredriksson M, Nordstrom I, Holmgren J. Cholera toxin and its B subunit promote dendritic cell vaccination with different influences on Th1 and Th2 development. Infect Immun. 2003;71:1740–1747. doi: 10.1128/IAI.71.4.1740-1747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Frech SA, Kenney RT, Spyr CA, Lazar H, Viret JF, Herzog C, Gluck R, Glenn GM. Improved immune responses to influenza vaccination in the elderly using an immunostimulant patch. Vaccine. 2005;23:946–950. doi: 10.1016/j.vaccine.2004.06.036. [DOI] [PubMed] [Google Scholar]
- Ghochikyan A, Vasilevko V, Petrushina I, Movsesyan N, Babikyan D, Tian W, Sadzikava N, Ross TM, Head E, Cribbs DH, Agadjanyan MG. Generation and characterization of the humoral immune response to DNA immunization with a chimeric beta-amyloid-interleukin-4 minigene. Eur J Immunol. 2003;33:3232–3241. doi: 10.1002/eji.200324000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn GM, Kenney RT, Ellingsworth LR, Frech SA, Hammond SA, Zoeteweij JP. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev Vaccines. 2003;2:253–267. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- Glenn GM, Rao M, Matyas GR, Alving CR. Skin immunization made possible by cholera toxin. Nature. 1998;391:851–852. doi: 10.1038/36014. [DOI] [PubMed] [Google Scholar]
- Guebre-Xabier M, Hammond SA, Ellingsworth LR, Glenn GM. Immunostimulant patch enhances immune responses to influenza virus vaccine in aged mice. J Virol. 2004;78:7610–7618. doi: 10.1128/JVI.78.14.7610-7618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guebre-Xabier M, Hammondx SA, Epperson DE, Yu J, Ellingsworth L, Glenn GM. Immunostimulant patch containing heat-labile enterotoxin from Escherichia coli enhances immune responses to injected influenza virus vaccine through activation of skin dendritic cells. J Virol. 2003;77:5218–5225. doi: 10.1128/JVI.77.9.5218-5225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–666. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke A. DNA immunization—a new chance in vaccine research? Med Microbiol Immunol. 2002;191:187–190. doi: 10.1007/s00430-002-0144-z. [DOI] [PubMed] [Google Scholar]
- Kieber-Emmons T, Monzavi-Karbassi B, Wang B, Luo P, Weiner DB. Cutting edge: DNA immunization with minigenes of carbohydrate mimotopes induce functional anti-carbohydrate antibody response. J Immunol. 2000;165:623–627. doi: 10.4049/jimmunol.165.2.623. [DOI] [PubMed] [Google Scholar]
- Laddy DJ, Weiner DB. From plasmids to protection: a review of DNA vaccines against infectious diseases. Int Rev Immunol. 2006;25:99–123. doi: 10.1080/08830180600785827. [DOI] [PubMed] [Google Scholar]
- Lavelle EC, Jarnicki A, McNeela E, Armstrong ME, Higgins SC, Leavy O, Mills KH. Effects of cholera toxin on innate and adaptive immunity and its application as an immunomodulatory agent. J Leukoc Biol. 2004;75:756–763. doi: 10.1189/jlb.1103534. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, Chattergoon MA, Baine Y, Higgins TJ, Ciccarelli RB, Coney LR, Ginsberg RS, Weiner DB. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Green TD, Bright RA, Ross TM. Induction of heterosubtypic immunity to influenza A virus using a DNA vaccine expressing hemagglutinin-C3d fusion proteins. Vaccine. 2003;21:902–914. doi: 10.1016/s0264-410x(02)00539-x. [DOI] [PubMed] [Google Scholar]
- Nikolic WV, Bai Y, Obregon D, Hou H, Mori T, Zeng J, Ehrhart J, Shytle RD, Giunta B, Morgan D, Town T, Tan J. Transcutaneous beta-amyloid immunization reduces cerebral beta-amyloid deposits without T cell infiltration and microhemorrhage. Proc Natl Acad Sci USA. 2007;104:2507–2512. doi: 10.1073/pnas.0609377104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke LM, Tooze R, Turner M, Sandoval DM, Carter RH, Tybulewicz VL, Fearon DT. CD19 as a membrane-anchored adaptor protein of B lymphocytes: costimulation of lipid and protein kinases by recruitment of Vav. Immunity. 1998;8:635–645. doi: 10.1016/s1074-7613(00)80568-3. [DOI] [PubMed] [Google Scholar]
- Peachman KK, Rao M, Alving CR. Immunization with DNA through the skin. Methods. 2003;31:232–242. doi: 10.1016/s1046-2023(03)00137-3. [DOI] [PubMed] [Google Scholar]
- Robinson HL, Boyle CA, Feltquate DM, Morin MJ, Santoro JC, Webster RG. DNA immunization for influenza virus: studies using hemagglutinin- and nucleoprotein-expressing DNAs. J Infect Dis. 1997;176 Suppl 1:S50–S55. doi: 10.1086/514176. [DOI] [PubMed] [Google Scholar]
- Ross TM, Xu Y, Bright RA, Robinson HL. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol. 2000;1:127–131. doi: 10.1038/77802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SB, Silver PB, Wang P, Chan CC, Caspi RR. Cholera toxin prevents Th1-mediated autoimmune disease by inducing immune deviation. J Immunol. 2004;173:755–761. doi: 10.4049/jimmunol.173.2.755. [DOI] [PubMed] [Google Scholar]
- van Rooij EM, Haagmans BL, de Visser YE, de Bruin MG, Boersma W, Bianchi AT. Effect of vaccination route and composition of DNA vaccine on the induction of protective immunity against pseudorabies infection in pigs. Vet Immunol Immunopathol. 1998;66:113–126. doi: 10.1016/s0165-2427(98)00186-x. [DOI] [PubMed] [Google Scholar]
- Wang H, Hosiawa KA, Min W, Yang J, Zhang X, Garcia B, Ichim TE, Zhou D, Lian D, Kelvin DJ, Zhong R. Cytokines regulate the pattern of rejection and susceptibility to cyclosporine therapy in different mouse recipient strains after cardiac allografting. J Immunol. 2003;171:3823–3836. doi: 10.4049/jimmunol.171.7.3823. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Ross TM, Tamura S, Ichinohe T, Ito S, Takahashi H, Sawa H, Chiba J, Kurata T, Sata T, Hasegawa H. Protection against influenza virus infection by intranasal administration of C3d-fused hemagglutinin. Vaccine. 2003;21:4532–4538. doi: 10.1016/s0264-410x(03)00510-3. [DOI] [PubMed] [Google Scholar]
- Wheeler M, Cortez-Gonzalez X, Frazzi R, Zanetti M. Ex vivo programming of antigen-presenting B lymphocytes: considerations on DNA uptake and cell activation. Int Rev Immunol. 2006;25:83–97. doi: 10.1080/08830180600743131. [DOI] [PubMed] [Google Scholar]


