SUMMARY
Starvation induces autophagy to preserve cellular homeostasis in virtually all eukaryotic organisms. However, the mechanisms by which starvation induces autophagy are not completely understood. In mammalian cells, the anti-apoptotic protein, Bcl-2, binds to Beclin 1 during non-starvation conditions, and inhibits its autophagy function. Here we show that starvation induces phosphorylation of cellular Bcl-2 at residues T69, S70, and S87 of the non-structured loop, Bcl-2 dissociation from Beclin 1, and autophagy activation. In contrast, viral Bcl-2, which lacks the phosphorylation site-containing non-structured loop, fails to dissociate from Beclin 1 during starvation. Furthermore, the stress-activated signaling molecule, c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, mediates starvation-induced Bcl-2 phosphorylation, Bcl-2 dissociation from Beclin 1, and autophagy activation. Together, our findings demonstrate that JNK1-mediated multi-site phosphorylation of Bcl-2 stimulates starvation-induced autophagy by disrupting the Bcl-2/Beclin 1 complex. These findings define a mechanism that cells use to regulate autophagic activity in response to nutrient status.
INTRODUCTION
Autophagy is an evolutionarily conserved cellular pathway in which the cell sequesters cytoplasmic contents in a double-membrane vesicle and delivers them to the lysosome for degradation (Levine and Klionsky, 2004). This pathway maintains cellular energy homeostasis during starvation; contributes to tissue remodeling during development; and removes harmful or superfluous cellular organelles, aggregate-prone proteins, and intracellular pathogens. The aberrant regulation of autophagy also contributes to a number of diseases (Levine and Kroemer, 2008).
An essential function of autophagy is cellular adaptation to nutritional stress. Following autophagic degradation of sequestered cytoplasmic cargo, the breakdown products are released into the cytoplasm where they can be recycled to maintain ATP energy production and macromolecular synthesis. Autophagy is implicated in adaptation to starvation in diverse organisms (Levine and Klionsky, 2004). One of the yeast genetic screens that identified the evolutionarily conserved autophagy (ATG) genes isolated mutants that died during nitrogen or carbon deprivation. ATG genes in higher eukaryotes are essential for survival during starvation in Dictyostelium, for survival during dauer diapause in C. elegans, for prevention of starvation-induced chlorosis in plants, and for survival during the neonatal starvation period in mice (Levine and Kroemer, 2008).
Given the fundamental role of autophagy in cellular and organismal adaptation to nutritional stress, an important question is how autophagy is stimulated by amino acid starvation. Several studies have focused on the role of insulin-dependent signaling and amino acids on the activation of mTOR, a potent inhibitor of autophagy (Meijer and Codogno, 2006). Less is known about how the absence of amino acids leads to autophagy stimulation. In response to changes in the intracellular ATP/AMP ratio, 5′ AMP-activated protein kinase (AMPK) is activated and phosphorylates TSC2, which increases its ability to inactivate mTOR (Meijer and Codogno, 2006). The eIF2α kinase, GCN2, senses low concentrations of amino acids, and along with eIF2α, is required for starvation-induced autophagy in yeast and mammalian cells (Talloczy et al., 2002). Other signaling molecules implicated in the control of starvation-induced autophagy include GTPases, calcium, MAPK family members, and ceramide (Meijer and Codogno, 2006).
Previous findings suggest that dissociation of Bcl-2 from Beclin 1 may also be an important mechanism for activating autophagy in response to starvation (Pattingre et al., 2005). Beclin 1, the mammalian orthologue of yeast Atg6, is part of a complex with class III PI3K and other proteins, including UVRAG, Ambra-1, Bif-1, and anti-apoptotic Bcl-2 family members (reviewed in Levine and Kroemer, 2008). Beclin 1-associated class III PI3K activity stimulates autophagy, presumably by mediating the localization of other autophagy proteins to the pre-autophagosomal membrane. The autophagy function of the Beclin 1-class III PI3K complex is activated by UVRAG, Ambra-1, and Bif-1, and inhibited by Bcl-2 and Bcl-xL. Previously, we found that nutrient conditions regulate the interaction between endogenous Bcl-2 and Beclin 1 (Pattingre et al., 2005). When autophagy is induced by nutrient deprivation, Bcl-2 binding to Beclin 1 is minimal; when autophagy is inhibited by nutrient excess, Bcl-2 binding to Beclin 1 is maximal.
The mechanism(s) by which nutrient conditions regulate the interaction between Bcl-2 and Beclin 1 are unknown. Since endoplasmic reticulum (ER)-localized Bcl-2 inhibits autophagy (Pattingre et al., 2005), and phosphorylated Bcl-2 localizes predominantly to the ER (Bassik et al., 2004), one possibility is that Bcl-2 is a target for TOR or other autophagy-inhibitory signaling kinases involved in nutrient sensing. According to such a model, Bcl-2 phosphorylation would promote binding to Beclin 1 and autophagy inhibition. However, viral Bcl-2 (vBcl-2) encoded by Kaposi’s sarcoma-associated herpesvirus (KSHV) inhibits Beclin 1-dependent autophagy as effectively as cellular Bcl-2 (Pattingre et al., 2005), and viral Bcl-2 lacks the non-structured loop of cellular Bcl-2 that contains regulatory phosphorylation sites (Huang et al., 2002). This suggests phosphorylation of Bcl-2-like family members may not be required for binding to Beclin 1 and autophagy inhibition. Further, based on studies of Bcl-2 phosphorylation in apoptotic regulation (Blagosklonny, 2001; Chang et al., 1997), it is also plausible that Bcl-2 phosphorylation might inhibit binding to Beclin 1, thereby promoting autophagy. This latter model is attractive in view of recent structural evidence that the Bcl-2 binding site of Beclin 1 is a BH3 domain (Maiuri et al., 2007; Oberstein et al., 2007), since phosphorylation of Bcl-2 blocks its binding to other BH3-only containing proteins (Bassik et al., 2004).
We examined whether Bcl-2 phosphorylation regulates its nutrient status-dependent interaction with Beclin 1 and anti-autophagy function. Our results indicate that Bcl-2 phosphorylation by the stress-activated signaling molecule, c-Jun N-terminal kinase 1 (JNK1), is required for its starvation-induced dissociation from Beclin 1 and autophagy activation.
RESULTS
Starvation Regulates the Binding of Cellular But Not Viral Bcl-2 to Beclin 1
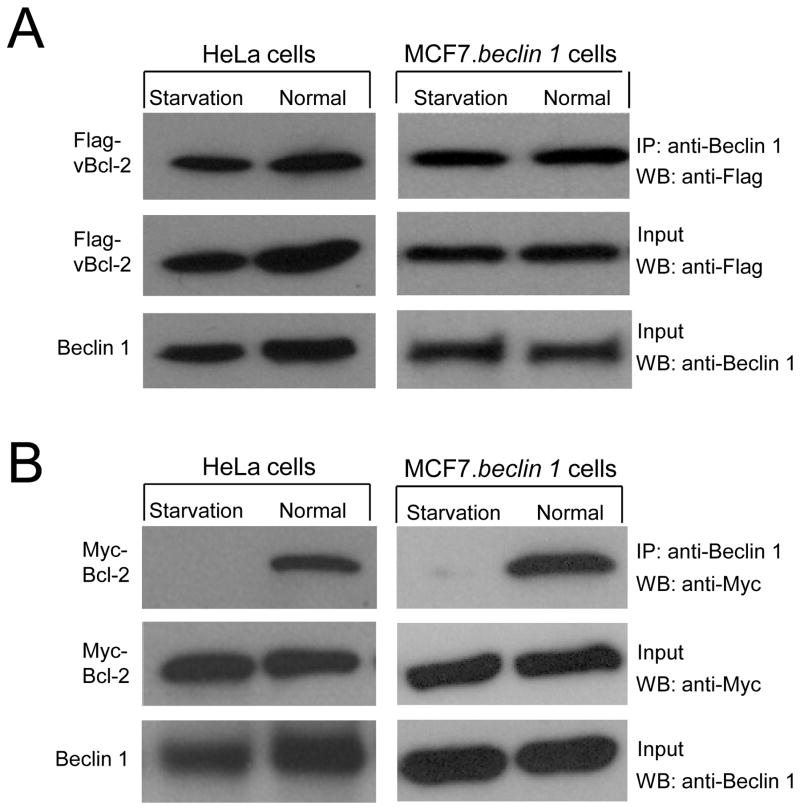
Previously, we showed that endogenous Bcl-2 binding to Beclin 1 is regulated by nutrient conditions in HeLa cells (Pattingre et al., 2005). We examined whether the Beclin 1 binding activity of KSHV vBcl-2 is also regulated by nutrient conditions (Figure 1A). In HeLa cells transfected with KSHV vBcl-2, we found no significant difference in the levels of vBcl-2 that co-immunoprecipitated with Beclin 1 when cells were grown in normal media or following 4 hours of nutrient deprivation. Similar results were also observed in MCF7 cells stably transfected with beclin 1 (MCF7.beclin 1 cells), a cell line used extensively for studies of Beclin 1-dependent autophagy (reviewed in Orvedahl et al., 2007). In contrast, in both HeLa cells and MCF7.beclin 1 cells transfected with cellular Bcl-2, less cellular Bcl-2 co-immunprecpitated with Beclin 1 during starvation conditions (Figure 1B). As reported previously, this starvation-induced dissociation of the Bcl-2-Beclin 1 complex is also seen with endogenous proteins in HeLa cells (Figure 2B). These observations confirm prior findings that starvation induces dissociation of the cellular Bcl-2-Beclin 1 complex, and demonstrate that viral Bcl-2 escapes this starvation-induced regulation of binding to Beclin 1.
Figure 1. Starvation Regulates the Interaction between Cellular but not Viral Bcl-2 and Beclin 1.
(A) Co-immunoprecipitation of Flag epitope-tagged KSHV vBcl-2 with Beclin 1 in HeLa cells or MCF7.beclin 1 cells transfected with a plasmid expressing Flag-vBcl-2, and grown either in normal media (normal) or in HBSS for four hours (starvation).
(B) Co-immunoprecipitation of Myc epitope-tagged cellular Bcl-2 with Beclin 1 in HeLa cells or MCF7.beclin 1 cells transfected with a plasmid expressing Myc-Bcl-2, and grown either in normal media (normal) or in HBSS for four hours (starvation).
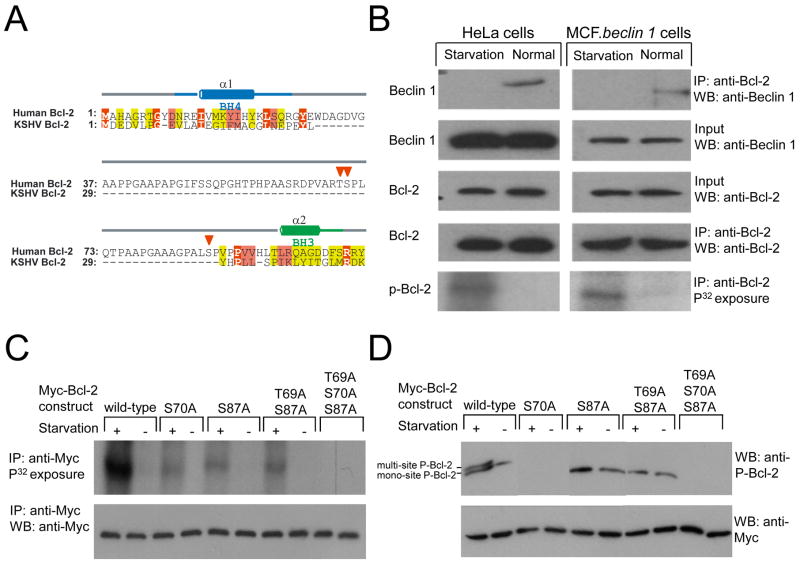
Figure 2. Starvation Stimulates Bcl-2 Multi-Site Phosphorylation.
(A) Structure-based sequence alignment of human Bcl-2 and KSHV vBcl-2. Residue numbers for each sequence are indicated in alignment block. Yellow, pink and red backgrounds indicate increasing sequence conservation, with identical residues highlighted in bold white letter. α-helices and coil secondary structures, color-coded by BH domain are indicated above each alignment. Red triangles indicate phosphorylation sites within the non-structured loop of human Bcl-2.
(B–C) Detection by P32 metabolic labeling of phosphorylated endogenous Bcl-2 in HeLa cells and MCF7.beclin 1 cells (B) or phosphorylated Bcl-2 in MCF7.beclin 1 cells transfected with wild-type or mutant forms of Myc-Bcl-2 (C). Cells were grown in normal media (starvation -) or HBSS for four hours (starvation +) prior to lysis, immunoprecipitated with anti-Bcl-2 (B) or anti-Myc (C), separated by SDS-PAGE, and subjected to either autoradiography or Western blot analysis with the indicated antibody.
(D) Detection of phosphorylated Bcl-2 using a phospho-specific anti-human Bcl-2 (Ser70) antibody (top panel) in MCF7.beclin 1 cells transfected with wild-type or mutant forms of Myc-Bcl-2. Cells were grown in normal media (starvation -) or HBSS for four hours (starvation +) prior to lysis. The faster migration band represents mono-site phosphorylation on serine 70 of Bcl-2 and the slower migration band (arrow) indicates multi-site phosphorylation.
Starvation Stimulates Multi-Site Phosphorylation Within the Cellular Bcl-2 Non-Structured Loop
We reasoned that a structural comparison of cellular Bcl-2 (which is subject to starvation-mediated regulation of binding to Beclin 1) and KSHV v-Bcl-2 (which is not subject to starvation-mediated regulation of binding to Beclin 1) might provide clues about the molecular mechanism(s) governing the regulation of Bcl-2/Beclin 1 binding. Cellular Bcl-2 contains a 58 amino acid non-structured loop between the BH4 and BH3 domain that is lacking in KSHV vBcl-2 (Figure 2A). This loop contains three major phosphorylation sites, T69, S70, and S87 (Blagosklonny, 2001). Therefore, we postulated that phosphorylation of one or more of these Bcl-2 sites may regulate binding to Beclin 1.
To investigate this, we examined whether endogenous Bcl-2 is phosphorylated in a nutrient status-dependent manner (Figure 2B). Following metabolic labeling with P32 and immunoprecipitation with an anti-Bcl-2 antibody, minimal or no phosphorylated Bcl-2 could be detected in HeLa and MCF7.beclin 1 cells in normal growth conditions. In contrast, during starvation, increased Bcl-2 phosphorylation was detected in both HeLa and MCF7.beclin 1 cells. In parallel with increased Bcl-2 phosphorylation, Beclin 1 failed to co-immunoprecipitate with Bcl-2 during starvation conditions. These data suggest that the starvation-induced phosphorylated form of endogenous Bcl-2 does not bind to Beclin 1.
Next we sought to map the starvation-induced phosphorylation sites of Bcl-2 by expressing wild-type and mutant forms of Myc-tagged Bcl-2 in MCF7.beclin 1 cells (Figure 2C). Similar to endogenous Bcl-2, strong phosphorylation of Myc-Bcl-2 was detected during starvation. Alanine substitutions of one or two potential phosphorylation sites (e.g. S70A, S87A, T69AS87A) in the non-structured loop decreased but did not completely abrogate starvation-induced Bcl-2 phosphorylation. In contrast, simultaneous alanine substitutions at three phosphorylation sites, T69, S70, and S87, completely blocked starvation-induced Bcl-2 phosphorylation. Thus, Bcl-2 multi-site phosphorylation occurs at residues T69, S70, and S87 during nutrient deprivation in MCF7.beclin 1 cells.
We confirmed these results using a phospho-specific antibody that detects serine-70 phosphorylation of human Bcl-2 (Figure 2D). The phospho-Ser70 specific Bcl-2 antibody fails to detect a band in Bcl-2 S70A mutant-expressing cells, confirming its specificity. During normal conditions, one band was detected in cells expressing wild-type Bcl-2, whereas during starvation, a second slower migration band was also detected. Previous studies have indicated that this slower migration band represents Bcl-2 multi-site phosphorylation while the faster migration band represents Bcl-2 S70 mono-site phosphorylation (Bassik et al., 2004; Yamamoto et al., 1999). In MCF7.beclin 1 cells expressing Bcl-2 T69A/S87, the slower migration band is absent during starvation, confirming that starvation induces multi-site phosphorylation, including of residues T69 and S87 within the non-structured Bcl-2 loop.
Multi-site Phosphorylation in the Bcl-2 Non-Structured Loop is Required for Nutrient Regulation of Beclin 1 Binding
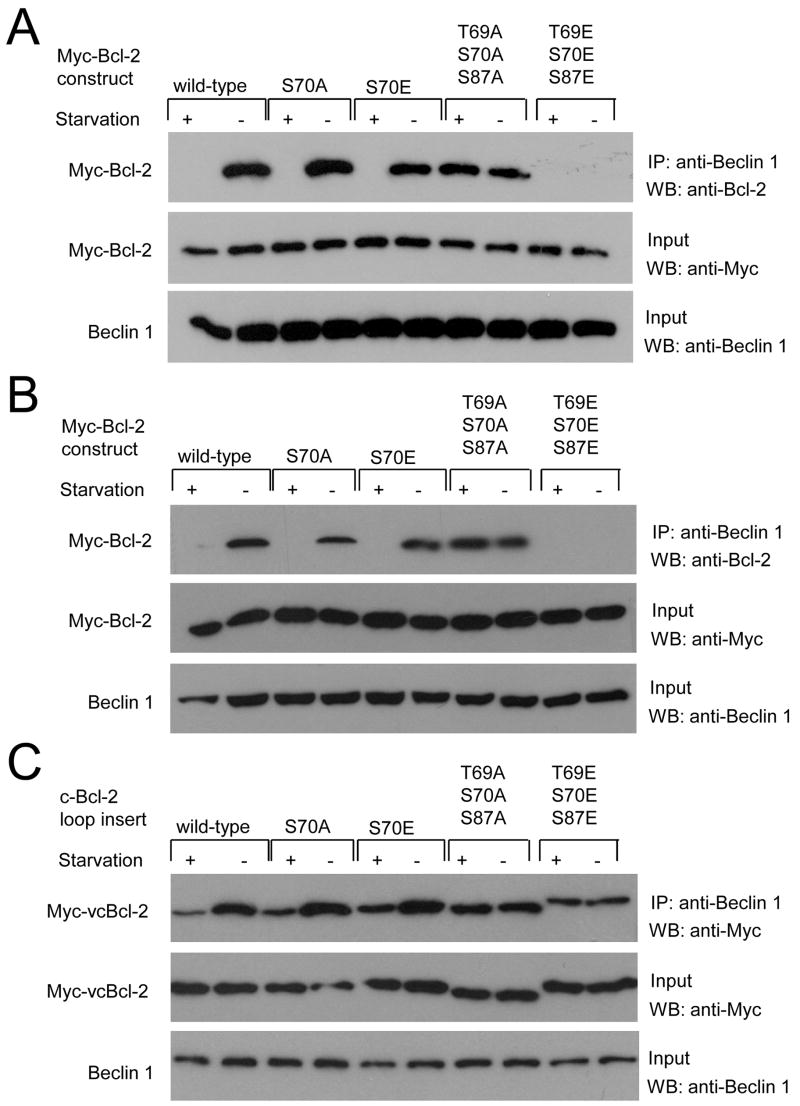
To further investigate the role of Bcl-2 multi-site phosphorylation in binding to Beclin 1 and autophagy regulation, we studied Bcl-2 mutants that either mimic (e.g. S70E or T69E/S70E/S87E) or abrogate (e.g. S70A or T69A/S70A/S87A) mono- or multi-site phosphorylation. During normal growth conditions, non-phosphorylatable mono-site Bcl-2 S70A and multi-site T69/S70A/S87A (AAA) mutants, and the mono-site phosphomimetic S70E mutant co-immunoprecipitate with Beclin 1 in both MCF7.beclin 1 cells (Figure 3A) and in HeLa cells (Figure 3B). However, during these conditions, the multi-site phosphomimetic Bcl-2 T69E/S70E/S87E (EEE) mutant fails to co-immumunoprecipitate with Beclin 1, suggesting that Bcl-2 multi-site phosphorylation blocks its interaction with Beclin 1. Also, the non-phosphorylatable Bcl-2 AAA mutant, unlike wild-type Bcl-2 or the mono-phosphorylation site S70A mutant, co-immunoprecipitates with Beclin 1 as strongly during starvation conditions as during nutrient rich conditions. This failure of a non-phosphorylatable Bcl-2 AAA mutant to dissociate with Beclin 1 during starvation is not limited to transient transfection assays. In previously characterized bcl-2−/− murine embryonic fibroblasts (MEFs) reconstituted with comparable levels of stably expressed human wild-type Bcl-2 (WT Bcl-2 MEFs) or AAA mutant Bcl-2 (AAA Bcl-2 MEFs) (Bassik et al., 2004), we found that starvation-induced phosphorylation of Bcl-2 and dissociation of the Bcl-2/Beclin 1 complex occurred in WT Bcl-2 but not in AAA Bcl-2 MEFs (Figure 7A). Together, these data indicate that starvation induces dissociation of the Bcl-2/Beclin 1 complex by multi-site phosphorylation of the Bcl-2 non-structured loop.
Figure 3. Phosphorylation Site Mutations in Bcl-2 Alter the Regulation of Bcl-2/Beclin 1 Binding.
(A and B) Co-immunoprecipitation of Bcl-2 with Beclin 1 in MCF7.beclin 1 cells (A) or HeLa cells (B) transfected with plasmids expressing wild-type or designated mutant Myc-tagged Bcl-2 and grown in normal media (starvation -) or HBSS for four hours (starvation +).
(C) Co-immunoprecipitation of chimeric viral/cellular Bcl-2 constructs with Beclin 1 in MCF7.beclin 1 cells transfected with plasmids expressing chimeras with wild-type or indicated mutant cellular Bcl-2 loop inserts and grown either in normal media (starvation -) or in HBSS for four hours (starvation +).
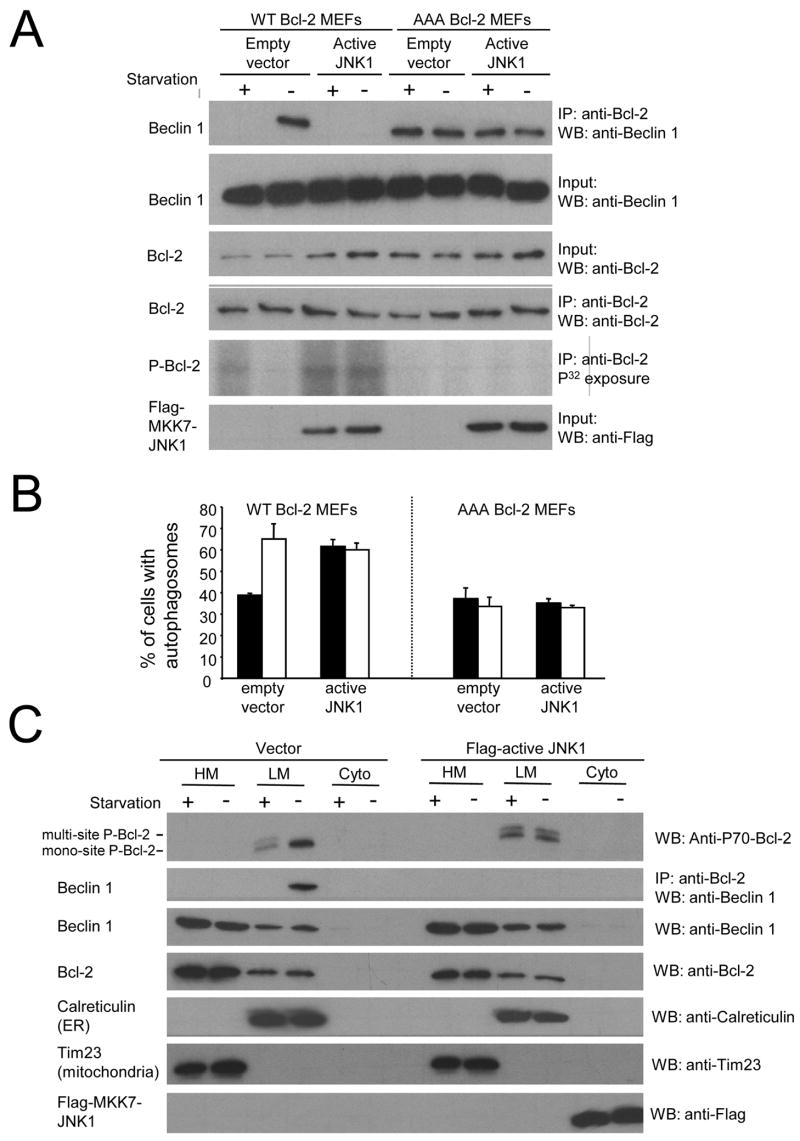
Figure 7. Starvation and JNK1 Regulate Autophagy Through Multi-Site Phosphorylation of the ER-Localized Pool of Bcl-2 and Disruption of the ER Bcl-2/Beclin 1 Complex.
(A) Effects of nutrient conditions and constitutively active JNK1 on Bcl-2 phosphorylation (fifth panel) and Beclin 1 co-immunoprecipitation with Bcl-2 (upper panel) in WT Bcl-2 MEFs or AAA Bcl-2 MEFs. Growth in normal media is represented as “starvation-“ and growth in HBSS is represented as “starvation+”. Other panels represent the total amount of Beclin 1 (second panel), Bcl-2 (third panel), or Flag-MKK7-JNK1 (sixth panel) detected in cell lysates by Western blot analysis with the indicated antibody or the total amount of Bcl-2 immunoprecipitated by anti-Bcl-2 (fourth panel).
(B) Light microscopic quantitation of autophagy in WT bcl-2 MEFs and AAA Bcl-2 MEFs co-transfected with GFP-LC3 and plasmid indicated below x axis. Solid bars represent growth in normal media and open bars represent growth in HBSS for four hours (nutrient starvation). Results shown represent mean ± SEM for triplicate samples of greater than 100 cells per sample. Similar results were obtained in three independent experiments.
(C) Effects of nutrient conditions and constitutively active JNK1 on Bcl-2 phosphorylation (top panel) and Beclin 1 co-immunoprecipitation with Bcl-2 (second panel) in heavy membrane (HM, mitochondria), light membrane (LM, ER) or cytosol (Cyto) fractions in WT Bcl-2 MEFs. Growth in normal media is represented as “starvation-“ and growth in HBSS is represented as “starvation+”. Other panels represent the total amount of Beclin 1 (third panel), Bcl-2 (fourth panel), Flag-MKK7-JNK1 (bottom panel), Calreticulin (ER marker, fifth panel) or Tim 23 (mitochondria marker, sixth panel) detected in cell lysates by Western blot analysis with the indicated antibody.
To confirm this hypothesis, we constructed chimeric viral/cellular Bcl-2 constructs in which we substituted either wild-type or S70A, S70E, AAA, or EEE mutant forms of the non-structured loop of cellular Bcl-2 for amino acids 24-34 of viral Bcl-2 (Figure 3C). The insertion of wild-type, S70A, or S70E Bcl-2 loop partially restored starvation-induced decreases in viral Bcl-2 binding to Beclin 1. The nonphosphorylatable AAA mutant loop did not restore nutrient-dependent binding, and the triple phosphomimetic EEE mutant loop had decreased binding to Beclin 1 during normal growth conditions. Thus, viral Bcl-2 can behave similarly to cellular Bcl-2 with respect to nutrient-dependent regulation of Beclin 1 binding if engineered to express the cellular Bcl-2 non-structured loop containing the phosphorylation sites T69, S70, and S87. These data further demonstrate the importance of multi-site phosphorylation of this loop in mediating starvation-induced dissociation from Beclin 1.
Multi-Site Phosphorylation in the Bcl-2 Non-Structured Loop Blocks its Anti-Autophagy Function
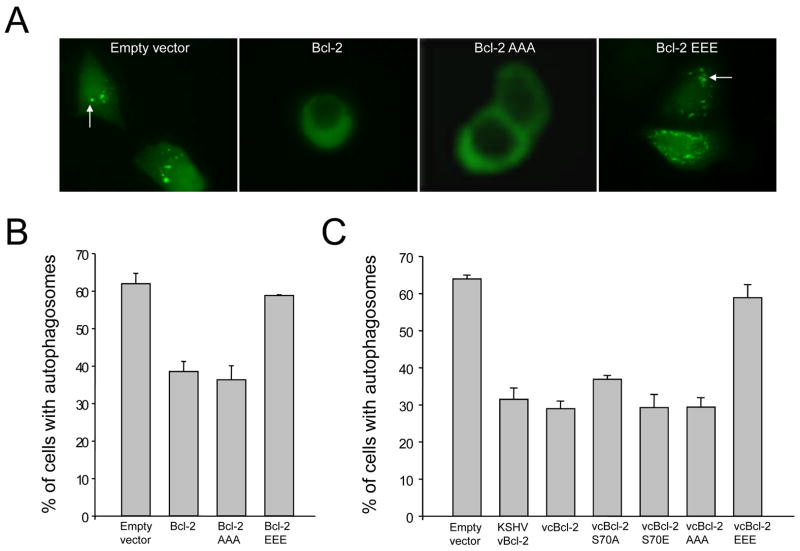
Previously, we showed that Bcl-2 inhibits autophagy through its interaction with Beclin 1 (Pattingre et al., 2005). Our data above indicate that multi-site phosphorylation of the non-structured loop blocks Bcl-2 binding to Beclin 1 during starvation. Therefore, we predicted that Bcl-2 multi-site phosphorylation would inhibit its anti-autophagy function. To test this, we compared the anti-autophagy activity of wild-type Bcl-2, Bcl-2 AAA, and Bcl-2 EEE mutants using the GFP-LC3 light microscopy assay which distinguishes between autophagy-inactive cells (with diffuse GFP-LC3 staining) and autophagy active cells (with punctate GFP-LC3 staining) (Klionsky et al., 2008) (Figure 4). We found that the non-phosphorylatable Bcl-2 AAA mutant inhibited starvation-induced autophagy as effectively as wild-type Bcl-2 (Figure 4A–B). In contrast, the phosphomimetic Bcl-2 EEE mutant was defective in the inhibition of starvation-induced autophagy. Similarly, introduction of the cellular Bcl-2 S70A, S70E or AAA mutant loops into viral Bcl-2 had no effect on the ability of viral Bcl-2 to inhibit autophagy, whereas the cellular Bcl-2 EEE mutant loop completely blocked the ability of viral Bcl-2 to inhibit starvation-induced autophagy (Figure 4C). Together, these findings indicate that Bcl-2 phosphorylation abrogates its anti-autophagy function, likely by mediating its dissociation from Beclin 1.
Figure 4. Phosphorylation Site Mutations in Bcl-2 Alter the Regulation of Autophagy.
(A) Representative images of GFP-LC3 staining during starvation in MC7.beclin 1 cells co-transfected with GFP-LC3 and indicated plasmid. Arrows denote representative cells containing GFP-LC3 dots (i.e. autophagosomal structures).
(B and C) Light microscopic quantitation of autophagy in MCF7.beclin 1 cells co-transfected with GFP-LC3 and plasmid indicated below x axis. In (B) plasmids expressed wild-type or indicated mutant cellular Bcl-2. In (C) plasmids express a chimeric viral/cellular Bcl-2 in which amino acids 24-34 of vBcl-2 are replaced with amino acids 25-95 from wild-type or indicated mutant cellular Bcl-2. Results shown represent mean ± SEM for triplicate samples of greater than 100 cells per sample. Similar results were obtained in three independent experiments.
JNK1 is the Kinase Responsible for Starvation-Induced Bcl-2 Multi-Site Phosphorylation, Disruption of the Bcl-2/Beclin 1 Complex, and Autophagy Activation
We next sought to identify the upstream kinase responsible for starvation-induced phosphorylation of Bcl-2 and disruption of its Beclin 1-binding and autophagy-inhibitory activity. Several kinases have been reported to phosphorylate Bcl-2, but only two kinases, both members of the mitogen-activated protein kinase (MAPK) superfamily, are known to phosphorylate Bcl-2 at multiple residues. C-jun N-terminal protein kinase (JNK) is the most frequently implicated Bcl-2 kinase and phosphorylates Bcl-2 at multiple sites in the non-structured loop, including residues T69, S70, and S87 (Maundrell et al., 1997; Yamamoto et al., 1999). The other stress-induced MAPK family member, P38, phosphorylates Bcl-2 at Ser87 and Thr56 but not at Thr69 or Ser70 within the non-structured loop (De Chiara et al., 2006). Therefore, we postulated that JNK may be the cellular kinase that regulates the anti-autophagy activity of Bcl-2 via multi-site phosphorylation,
First, we evaluated whether JNK can be activated by starvation in MCF7.beclin 1 cells using a phospho-specific antibody that recognizes dual site phosphorylation of different isoforms of JNK at Thr183 and Tyr185 (Figure 5A). No active JNK could be detected during growth in normal media, but active JNK was detected during starvation conditions, although total levels of JNK were equivalent in both conditions. In contrast to JNK, we did not detect starvation-induced activation of P38 (Figure 5B).
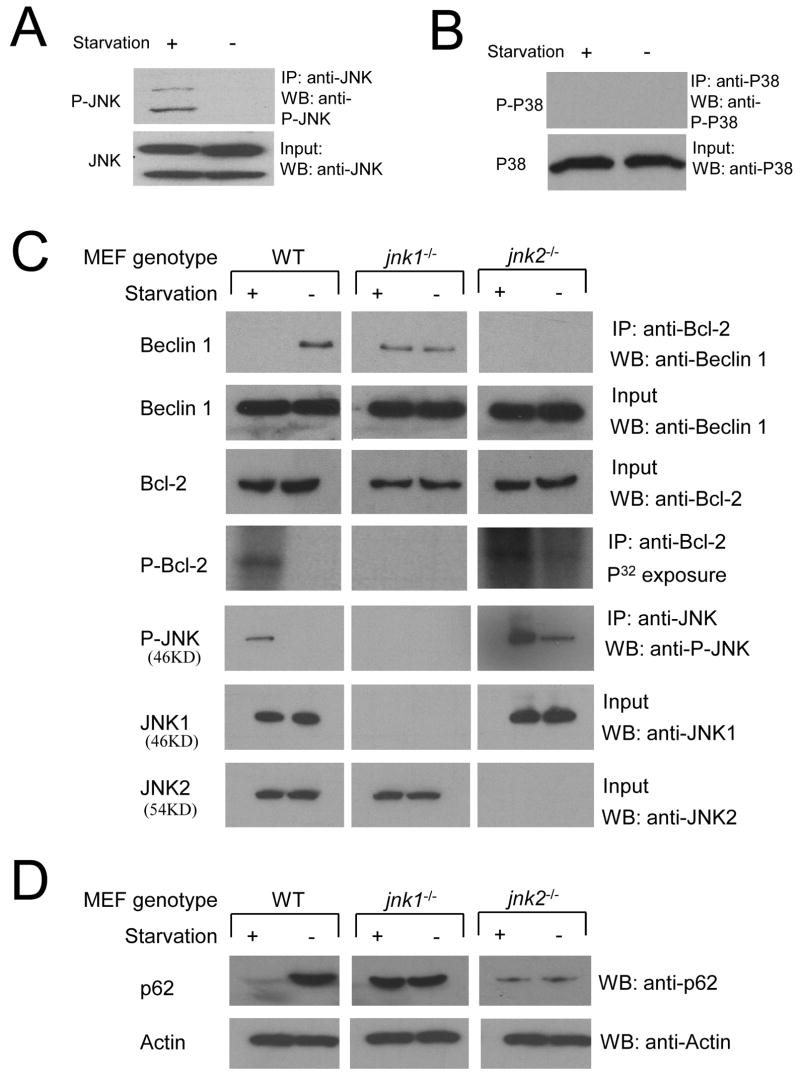
Figure 5. Endogenous jnk1 is Required for Starvation-Induced Bcl-2 Phosphorylation, Dissociation of the Bcl-2/Beclin 1 Complex, and Autophagy.
(A and B) Western blot analysis of active JNK (A) and active P38 (B) in MCF7.beclin 1 cells grown in normal media (starvation -) or in HBSS for four hours (starvation +), Active JNK was detected by immunoprecipitation using a polyclonal goat anti-JNK antibody, followed by Western blot analysis with a rabbit polyclonal Thr183/Tyr185 phosphorylation specific JNK antibody (A, upper panel). Active P38 was detected by a rabbit polyclonal phospho-P38 MAP kinase (Thr180/Tyr182) antibody (B, upper panel). Total JNK and P38 (A–B, lower panels) were detected by rabbit polyclonal JNK and p38 MAP kinase antibody, respectively.
(C) Comparison of Beclin 1 co-immunoprecipitation with Bcl-2 (top panel), Bcl-2 phosphorylation (fourth panel) and JNK phosphorylation (fifth panel) in wild-type (WT), jnk1−/− and jnk2−/− MEFS during growth in normal media (starvation -) or in HBSS for four hours (starvation +). Other panels represent the total amount of Beclin 1 (second panel), Bcl-2 (third panel), JNK1 (sixth panel) and JNK2 (bottom panel) detected in cell lysates by Western blot analysis with the indicated antibody.
(D) Comparison of p62/SQSTM1 levels in WT, jnk1−/− and jnk2−/− MEFS during growth in normal media (starvation -) or in HBSS for four hours (starvation +). Actin is shown as a loading control.
Next we evaluated whether endogenous JNK is required for starvation-induced Bcl-2 phosphorylation, using MEFs with targeted disruption of jnk genes, including jnk1 or jnk2 (Tournier et al. 2002) (Figure 5C). We found that, similar to our findings in MCF7.beclin 1 and HeLa cells, Bcl-2 phosphorylation can be detected by P32 metabolic labeling in wild-type MEFs during starvation, but not during growth in normal conditions. In contrast, starvation-induced Bcl-2 phosphorylation is completely blocked in jnk1−/− MEFs, indicating that endogenous jnk1 is essential for starvation-induced Bcl-2 phosphorylation. In jnk2−/− MEFs, Bcl-2 phosphorylation is observed during growth in normal media and increases further upon starvation. Since higher than normal levels of JNK activation have been observed in jnk2−/− mice (Tuncman et al. 2006), we hypothesized that this Bcl-2 phosphorylation in jnk2−/− MEFs may also reflect increased JNK activation. Indeed, in wild-type MEFs, we found JNK activation only during starvation, whereas JNK activation was observed during normal growth conditions in jnk2−/− MEFs (and further increased upon starvation). No JNK activation was observed in jnk1−/− MEFs in normal or starvation conditions, indicating that the hypercompensation by other jnk genes observed in jnk2−/− MEFs does not occur in jnk1−/− MEFs.
Beclin 1-Bcl-2 co-immunoprecipitation in wild-type, jnk1−/−, and jnk2−/− MEFs correlated inversely with Bcl-2 phosphorylation. In wild-type MEFs, as in other cell types described above, the non-phophosorylated form bound to Beclin 1 in normal growth conditions, whereas the phosphorylated form did not bind to Beclin 1 during starvation. In jnk1−/ MEFs, in which no Bcl-2 phosphorylation occurred during starvation, Bcl-2 retained the ability to co-immunoprecipitate Beclin 1. In jnk2−/− MEFs, in which Bcl-2 phosphorylation occurred during both normal conditions and starvation, Bcl-2 was unable to co-immunoprecipitate Beclin 1.
Since the viability of the jnk1−/− MEFs was poor following transient transfection of GFP-LC3 or empty control plasmids, we compared starvation-induced autophagy in WT jnk1−/−, and jnk2−/− MEFs using a biochemical method that detects the degradation of p62/SQSTM1, an adaptor protein that binds to LC3, is incorporated into the autophagosome, and degraded by the autophagolysosome (Klionsky et al., 2008) (Figure 5D). In WT MEFs, p62/SQSTM1 is rapidly degraded during starvation, indicating the induction of a successful autophagy response. In contrast, in jnk1−/− MEFs, the levels of p62/SQSTM1 remain unchanged during starvation, indicating the lack of normal starvation-induced autophagy. Further, in jnk2−/− MEFs, where no Bcl-2 binds to Beclin 1 during normal growth conditions (presumably due to increased JNK1 activation), there is a marked decrease in basal levels of p62, suggesting constitutive hyperactivation of the autophagy pathway.
These findings indicate that jnk1−/− but not jnk2−/− MEFs are deficient in starvation-induced Bcl-2 phosphorylation, dissociation of the Bcl-2/Beclin 1 complex and autophagy.
JNK1 Stimulates Starvation-Induced Autophagy Through Bcl-2 Multi-Site Phosphorylation
The above findings demonstrate an essential role for endogenous JNK1 in starvation-induced autophagy through a mechanism that involves Bcl-2 phosphorylation and disruption of the Bcl-2/Beclin 1 complex. We further confirmed the role of JNK1 in mediating autophagy through Bcl-2 multi-site phosphorylation, using cells that express human Bcl-2 (for which, unlike mouse Bcl-2, a phospho-specific antibody is available to detect Bcl-2 multi-site phosphorylation) and can be readily monitored for autophagosome formation with the GFP-LC3 assay.
We expressed a constitutively active JNK1 and a dominant negative mutant JNK1 in MCF7.beclin 1 cells (Figure 6A). We used a previously described construct in which JNK1 is fused to an upstream kinase, MKK7, that functions as a specific, constitutive activator of JNK1 (Lei et al., 2002; Zheng et al., 1999). The replacement of the tripeptide dual phosphorylation motif Thr-Pro-Tyr with Ala-Pro-Phe in the fusion protein MKK7-JNK1 generates a dominant negative JNK1 (Lei et al., 2002). Expression of constitutively active JNK1 in MCF7.beclin 1 cells results in constitutive Bcl-2 multi-site phosphorylation which is associated with a decrease in Bcl-2 co-immunoprecipitation with Beclin 1. In contrast, in MCF7.beclin 1 cells that express dominant negative JNK1, multi-site Bcl-2 phosphorylation is observed neither during normal growth conditions nor during starvation and Bcl-2 fails to dissociate from Beclin 1 during starvation.
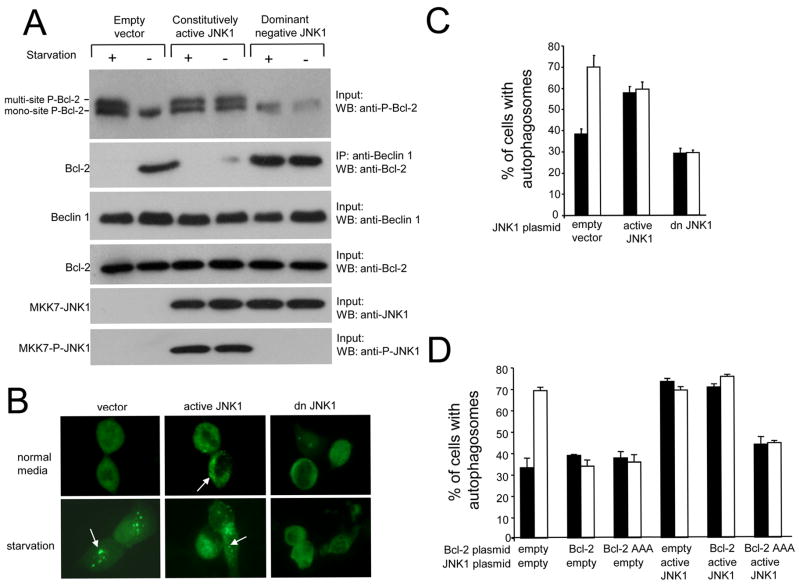
Figure 6. Constitutively Active and Dominant Negative JNK1 Regulate Bcl-2 Phosphorylation, Bcl-2 Binding to Beclin 1 and Starvation-Induced Autophagy.
(A) Effects of constitutively active JNK1 and dominant negative JNK1 on Bcl-2 phosphorylation (upper panel) and Bcl-2 co-immunoprecipitation with Beclin 1 (second panel) in MCF7.beclin 1 cells during growth in normal media (starvation -) or in HBSS for four hours (starvation +). Lower panels represent total amount of Beclin 1 (third panel), Bcl-2 (fourth panel), MKK7-JNK1 (fifth panel) or active MKK-JNK1 (MKK-P-JNK1) (sixth panel) detected in cell lysates by Western blot analysis with indicated antibody.
(B) Representative images of GFP-LC3 staining in MCF7.beclin 1 cells co-transfected with GFP-LC3 and indicated plasmid. Arrows denote representative cells containing GFP-LC3 dots (i.e. autophagosomal structures).
(C and D) Light microscopic quantitation of autophagy in MCF7.beclin 1 cells co-transfected with GFP-LC3 and plasmid(s) indicated below x axis. Results shown represent mean ± SEM for triplicate samples of greater than 100 cells per sample. Similar results were obtained in three independent experiments.
The effects of constitutively active and dominant negative JNK1 on autophagy correlate with effects on Bcl-2 phosphorylation and Beclin 1 binding (Figure 6B–C). Constitutively active JNK1 expression in MCF7.beclin 1 cells, which is associated with Bcl-2 multi-site phosphorylation and lack of Bcl-2 binding to Beclin 1 during growth in normal media, increases basal autophagy levels compared to empty vector control. In contrast, dominant negative JNK1 expression, which is associated with lack of Bcl-2 multi-site phosphorylation and the persistence of Bcl-2 binding to Beclin 1 during starvation conditions, blocks starvation-induced increases in autophagy. Together, these data confirm that JNK1 mediates Bcl-2 multi-site phosphorylation, Bcl-2 dissociation from Beclin 1, and autophagy induction in response to cellular starvation.
To further evaluate whether JNK1 stimulates autophagy by Bcl-2 multi-site phosphorylation, we examined the effects of forced expression of wild-type or the non-phosphorylatable AAA mutant Bcl-2 on autophagy induced by constitutively active JNK1 (Figure 6D). The starvation-induced increase in autophagy in MCF7.beclin 1 cells is blocked by overexpression of either wild-type or the non-phosphorylatable AAA mutant Bcl-2. In cells that express constitutively active JNK1 and have increased levels of autophagy during normal growth conditions, wild-type Bcl-2 forced expression fails to inhibit autophagy whereas AAA mutant Bcl-2 forced expression inhibits autophagy during growth in normal media and during starvation. Thus, the non-phosphorylatable AAA mutant Bcl-2 successfully blocks the ability of constitutively active JNK1 to stimulate autophagy.
Next, we used WT Bcl-2 or AAA Bcl-2 MEFs to further confirm that JNK1 phosphorylation of residues T69, S70, and S87, is critical for starvation-induced dissociation of Bcl-2 and Beclin 1 and autophagy activation. We found that constitutively active JNK1 induced phosphorylation of Bcl-2 and dissociation from Beclin 1 during normal growth conditions in WT Bcl-2 but not in AAA Bcl-2 MEFs (Figure 7A). Similarly, we found that constitutively active JNK1 increases autophagic activity during normal growth conditions in WT Bcl-2 MEFs (Figure 7B), whereas it is incapable of inducing autophagy in AAA Bcl-2 MEFs that lack endogenous phosphorylatable Bcl-2. Together, these observations suggest that JNK1 stimulates autophagy through a mechanism involving phosphorylation of Bcl-2 at residues T69, S70, and S87A.
JNK1 and Starvation Induce Multi-Site Phosphorylation of the ER-Localized Pool of Bcl-2
Previously, we showed that ER-localized Bcl-2, not mitochondrial-localized Bcl-2, inhibits Beclin 1-dependent autophagy. To determine which cellular pool of Bcl-2 is subject to JNK1-mediated phosphorylation, we performed subcellular fractionation of WT Bcl-2 MEFs transfected with empty vector or constitutively active JNK1 (Figure 7C), isolating heavy membrane fractions (labeled by the ER marker, calreticulin) and light membrane fractions (labeled by the mitochondrial marker, Tim23). In control transfected cells, the heavy membrane (ER) fraction contained mono-phosphorylated (S70) Bcl-2 during growth in normal conditions, and multi-site phosphorylated Bcl-2 during starvation. In cells transfected with active JNK1, multi-site Bcl-2 phosphorylation was observed in the ER fraction in both normal and starvation conditions. No Bcl-2 phosphorylation was detected under any conditions in the light membrane (mitochondrial) fraction. Thus, phosphorylated Bcl-2 is localized entirely in the ER-containing fraction, and multi-site phosphorylation of Bcl-2 likely occurs specifically at the ER during starvation or JNK1 activation. Upon immunoprecipitation of heavy membrane, light membrane, and cytoplasmic fractions with anti-Bcl-2, Beclin 1 was only isolated in a complex with the mono-phosphorylated Bcl-2 in the heavy membrane fraction during normal growth conditions. No Beclin 1 was isolated in multi-site phosphorylated Bcl-2 complexes in the heavy membrane fraction of control-transfected cells during starvation or active JNK1-transfected cells during starvation or normal nutrient conditions. The amount of Beclin 1 that co-immunoprecipitates with Bcl-2 appears similar to the amount of mono-phosphorylated Bcl-2 in the heavy membrane fraction, suggesting a stoichiometric correlation between mono-phosphorylated Bcl-2 and Beclin 1 complexed in the ER. Together, these data suggest a model in which JNK1-mediated phosphorylation of the ER pool of Bcl-2 during starvation disrupts its binding to Beclin 1, thereby releasing the inhibitory effects of Bcl-2 on autophagy.
DISCUSSION
Bcl-2 Multi-site Phosphorylation Regulates Induction of Starvation-Induced Autophagy
Our data demonstrate that multi-site phosphorylation within the non-structured loop of Bcl-2 stimulates starvation-induced autophagy by disrupting the Bcl-2/Beclin 1 complex. We found that starvation, a potent inducer of autophagy, induces phosphorylation of residues T69, S70, and S87 of cellular Bcl-2, which is required for dissociation of Bcl-2 from Beclin 1 during starvation. Viral Bcl-2, which lacks the non-structured loop and regulatory phosphorylation sites, is unable to dissociate from Beclin 1 during starvation. Moreover, a Bcl-2 mutant containing phosphomimetic mutations at T69, S70, and S87, is unable to bind to Beclin 1 and inhibit autophagy. Also, in response to starvation, Beclin 1 cannot be released from Bcl-2 and autophagy cannot be induced in cells that contain only a non-phosphorylatable Bcl-2 and lack endogenous wild-type Bcl-2. Previous studies have indicated that cellular and viral Bcl-2 family members inhibit autophagy and that the cellular Bcl-2/Beclin 1 complex dissociates during starvation (Maiuri et al., 2007; Pattingre et al., 2005). However, the molecular mechanism regulating the dissociation of Bcl-2 from Beclin 1 during starvation was unknown. Our findings indicate that this dissociation is regulated by starvation-induced Bcl-2 multi-site phosphorylation.
Bcl-2 has been studied most extensively as an anti-apoptotic protein, although it has been shown to be involved in other cellular processes, including cell cycle progression, calcineurin signaling, glucose homeostasis, transcriptional repression by p53, and more recently, in autophagy inhibition (Danial and Korsmeyer, 2004; Maiuri et al., 2007; Pattingre et al., 2005; Reed, 1998). The Bcl-2 protein has a long half-life, and its regulation at the level of protein expression is limited (Reed, 1996); therefore, post-translational modifications, including phosphorylation, may play an important role in regulating its activity (Blagosklonny, 2001). Previous studies demonstrated that Bcl-2 can be phosphorylated on specific residues within the non-structured loop that links the BH3 and BH4 domains in response to diverse stimuli, including chemotherapeutic drugs and growth factors (reviewed in Blagosklonny, 2001). There is controversy over the significance of Bcl-2 phosphorylation and its effects on apoptosis regulation (Blagosklonny, 2001). However, in most studies, phosphorylation of Bcl-2 is associated with inactivation, as deletion of the loop region or mutation of phosphorylation sites enhances its anti-apoptotic function (reviewed in Bassik et al., 2004). Our results show that Bcl-2 phosphorylation also inactivates its anti-autophagy function.
The effects of Bcl-2 phosphorylation on binding to Beclin 1 are consistent with previous results regarding the relationship between Bcl-2 phosphorylation and binding to BH3-domain containing proteins (Bassik et al., 2004). Phosphorylation of Bcl-2 inhibits binding to both multi-domain and BH3-only pro-apoptotic family members in a purified in vitro system. Furthermore, most phosphorylated Bcl-2 resides in the ER and in ER-enriched fractions, the non-phosphorylatable Bcl-2 AAA mutant co-precipitated substantially more BH3 pro-apoptotic family members than does wild-type Bcl-2. These observations led to the model that phosphorylation of ER-based Bcl-2 reduces binding to BH3 pro-apoptotic proteins. Of note, structural and functional analyses have recently identified Beclin 1 as a novel BH3-only protein that binds to Bcl-2 and Bcl-xL via its BH3 domain (Maiuri et al., 2007; Oberstein et al., 2007). Our prior work demonstrated that ER-localized Bcl-2 inhibits the autophagy function of Beclin 1 (Pattingre et al., 2005), and in this study we found that phosphorylation of ER-localized Bcl-2 regulates its binding to Beclin 1. Thus, there are parallels between the effects of Bcl-2 phosphorylation on apoptotic regulation and on autophagy regulation. In both cases, Bcl-2 phosphorylation interferes with its binding to BH3-containing proteins in the ER, including BH3-containing pro-apoptotic proteins (in the case of apoptosis regulation) and the BH3 containing pro-autophagy protein, Beclin 1 (in the case of autophagy regulation).
It is not yet clear how Bcl-2 phosphorylation disrupts binding to either Beclin 1 or BH3-containing pro-apoptotic family members. An important question is whether there are differential binding affinities of Bcl-2 to pro-apoptotic versus pro-autophagic BH3 proteins, and as a corollary, whether there is a hierarchical relationship between Bcl-2 phosphorylation and disruption of Bcl-2/Beclin 1 complexes versus disruption of Bcl-2/BH3 pro-apoptotic protein complexes. Since autophagy is activated as a cell survival mechanism during starvation (Levine and Klionsky, 2004), one prediction is that rapid Bcl-2 phosphorylation during starvation may occur initially to promote cell survival by disrupting the Bcl-2/Beclin 1 complex and activating autophagy. At a certain point when autophagy is incapable of further keeping cells alive and cells “commit” to death, Bcl-2 phosphorylation might then serve to inactivate its anti-apoptotic function.
Bcl-2 Phosphorylation, Disruption of the Bcl-2/Beclin 1 Complex, and Autophagy Stimulation During Starvation is Mediated by JNK1 Signaling
Our data show that the JNK1 signaling pathway is responsible for Bcl-2 multi-site phosphorylation during starvation, which leads to disruption of the Bcl-2/Beclin 1 complex and release of the inhibitory activity of Bcl-2 on Beclin 1-dependent autophagy. In cells lacking endogenous JNK1, starvation fails to induce Bcl-2 phosphorylation, disruption of the Bcl-2/Beclin 1 complex or autophagy stimulation. Further, constitutively active JNK1 results in Bcl-2 multi-site phosphorylation, disruption of the Bcl-2/Beclin 1 complex, and autophagy activation in cells grown in normal media whereas dominant negative JNK1 blocks the Bcl-2 multi-site phosphorylation, disruption of the Bcl-2/Beclin 1 complex, and autophagy activation that normally occurs in response to starvation. In addition, autophagy stimulation by constitutively active JNK1 is blocked by a non-phosphorylatable Bcl-2 mutant but not by wild-type Bcl-2. Together, these observations provide strong evidence that the JNK1 signaling pathway positively regulates autophagy by phosphorylation of Bcl-2 and release of its inhibitory effects on Beclin 1. Although there may be redundant actions of JNK1 and JNK2, our data add to the increasing evidence for JNK1/2-isoform specific roles (Bogoyevitch et al. 2006), since we failed to observe a similar role for JNK2 in the regulation of Bcl-2 phosphorylation, Bcl-2 binding to Beclin 1, and autophagy.
Our data suggest that an important physiological function of JNK1-mediated Bcl-2 phosphorylation/inactivation is stimulation of autophagy in response to starvation. Since autophagy is activated by starvation in lower eukaryotes such as yeast that lack Bcl-2, this regulatory mechanism has evolved more recently than the core autophagic machinery. One speculation is that, in metazoans, there is a need that does not exist in unicellular organisms for coordinate regulation of starvation-responses and autophagy with other pathways such as cellular proliferation, differentiation, development, inflammation, and apoptosis that are also regulated by JNK1 signaling (Liu and Lin, 2005;Weston and Davis, 2007).
The JNK1 signaling pathway has been shown to regulate autophagy in both Drosophila and mammalian cells in response not only to starvation, but also in response to ER stress, T cell receptor activation, growth factor withdrawal, cytokine stimulation (e.g. IL-2, TNFα), caspase inhibition, and treatment with neuronal excitotoxic stimuli (Borsello et al., 2003; He and Orvedahl, 2007; Jia et al., 2006; Li et al., 2006; Lu et al., 2007; Ogata et al., 2006; Yu et al., 2004). This diversity of stress stimuli that trigger JNK1-mediated autophagy is consistent with the hypothesis that autophagy regulation is intricately intertwined with numerous other cellular stress response programs mediated by JNK1 signaling. Despite the known link between JNK1 and autophagy induction, the mechanism by which JNK1 activation leads to autophagy induction has been undefined. In the present study, we show that, at least during starvation, the mechanism involves Bcl-2 phosphorylation and the release of Bcl-2’s inhibitory action on the essential autophagy protein, Beclin 1. It is not yet known whether the mechanism by which JNK1 activates autophagy in settings other than starvation are through its effects on Bcl-2 phosphorylation or through other downstream targets.
There is some evidence that temporal regulation of JNK1 activation may be a critical determinant of the cellular response. Early, transient JNK1 activation promotes cell survival whereas prolonged JNK1 activation can mediate apoptosis (Ventura et al., 2006). This finding is consistent with our observation that JNK1 activation and JNK1-mediated Bcl-2 phosphorylation promote autophagy - a cell survival pathway during starvation -even though these same signaling events may also contribute to cell death. In this study, we focused on the short-term effects of starvation (4 hours) when cells are healthy and no apoptotic programs are activated, which allowed us to dissociate effects of Bcl-2 and JNK1 signaling on autophagy regulation from their effects on apoptosis regulation. It will be interesting to test the prediction that short-term JNK1-mediated Bcl-2 phosphorylation may promote autophagy-dependent cell survival, whereas sustained JNK1-mediated Bcl-2 phosphorylation may promote apoptosis. Given that JNK1 has been shown to activate autophagy both in settings where autophagy promotes cell survival (e.g. starvation, ER stress) and in settings where autophagy leads to cell death (e.g. caspase inhibition, TNF-α stimulation, neuronal excitotoxic stimuli) (Borsello et al., 2003; Ogata et al., 2006; Yu et al., 2004), a related question is whether the magnitude and/or kinetics of JNK1-mediated Bcl-2 phosphorylation determines whether autophagy is pro-survival or pro-death.
In summary, we have identified a signaling mechanism that regulates starvation-induced autophagy in mammalian cells involving JNK1-mediated multi-site phosphorylation of Bcl-2 and disruption of the Bcl-2/Beclin 1 complex. Given the critical roles of both JNK1 and autophagy in enabling cells to successfully adapt to environmental stress, this mechanism may play an important role in diverse aspects of cellular and tissue homeostasis.
EXPERIMENTAL PROCEDURES
Please see Supplemental Experimental Procedures for information on plasmid constructions, cell transfections, metabolic labeling and phosphorylation detection, Western blot analyses, and details on antibodies, coimmunoprecipitation assays, and subcellular fractionation studies.
Plasmids
Flag-Bcl-2, Flag-v-Bcl-2, GFP-LC3 were described previously (Pattingre et al. 2005) and pCDNA3-Flag-MKK7-JNK1 (constitutively active JNK), and pcDNA3-Flag-MKK7-JNK1(APF) (dominant negative JNK1) were provided by R.J. Davis (Lei et al., 2002). The construction of mutant cellular and viral Bcl-2 plasmids is described in supplemental experimental procedures.
Antibodies
The antibodies used to detect Beclin 1, JNK1, JNK2, p62, Myc, Bcl-2, phosphorylated Bcl-2, phosphorylated JNK, phosphorylated p38, p62/SQSTM1 and Flag-epitope are described in the supplemental experimental procedures.
Cells
Cells used in this study include previously described HeLa and MCF7.beclin 1 cells (Pattingre et al., 2005), bcl-2−/− MEFs reconstituted with wild-type human Bcl-2 (WT Bcl-2 MEFS) or a non-phosphorylatable T69A, S70A, S87A mutant form of human Bcl-2 (AAA Bcl-2 MEFs) (Bassik et al., 2004), and jnk1−/− and jnk2−/− MEFs provided by R.J. Davis (Tournier et al. 2000). Cells were transfected as described in the supplemental experimental procedures.
Co-immunoprecipitation Assays
Immunoprecipitation of Beclin 1 and Bcl-2 were performed with a polyclonal goat anti-Beclin 1 antibody and a monoclonal anti-Bcl-2 antibody, respectively, using methods described in the supplemental experimental procedures.
Subcellular Fractionation Assays
WT Bcl-2 MEFs were fractionated into heavy membrane, light membrane, and cytosolic fractions as described in the supplemental experimental procedures.
Autophagy Assays
Autophagy was measured during growth in normal media or after 4 hours of starvation in Hanks Balanced Salt Solution (HBSS) by light microscopic quantitation of cells transfected with GFP-LC3 as described (Furuya et al., 2005) or by Western blot analysis of the levels of p62/SQSTM1.
Supplementary Material
Acknowledgments
This work was supported by NIH RO1 grants CA084254, CA108618, and AI051367 to B.L., and an Association pour la Recherche sur le Cancer grant n°4006 to S.P. We thank Roger Davis, Nika Danial, and Tamotsu Yoshimori for providing critical reagents and Cindy Jozefiak for administrative assistance. We are particularly grateful to Patrice Codogno for generous support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bassik MC, Scorrano L, Oakes SA, Pozzan T, Korsmeyer SJ. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J. 2004;23:1207–1216. doi: 10.1038/sj.emboj.7600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Unwinding the loop of Bcl-2 phosphorylation. Leukemia. 2001;15:869–874. doi: 10.1038/sj.leu.2402134. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA. The isoform-specific functions of the c-Jn N-terminal Kinases (JNKs): differences revealed by gene targeting. BioEssays. 2006;28:923–934. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- Borsello T, Croquelois K, Hornung JP, Clarke PG. N-methyl-D-aspartate-triggered neuronal death in organotypic hippocampal clutures is endocytic, autophagic and mediated by the c-Jun N-terminal kinase pathway. Eur J Neurosci. 2003;18:473–485. doi: 10.1046/j.1460-9568.2003.02757.x. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- De Chiara G, Marcocci ME, Torcia M, Lucibello M, Rosini P, Bonini P, Higashimoto Y, Damonte G, Armirotti A, Amodei S, et al. Bcl-2 phosphorylation by p38 MAPK: Identification of target sites and biologic consequences. J Biol Chem. 2006;281:21353–21361. doi: 10.1074/jbc.M511052200. [DOI] [PubMed] [Google Scholar]
- Furuya N, Yu J, Byfield M, Pattingre S, Levine B. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy. 2005;1:46–52. doi: 10.4161/auto.1.1.1542. [DOI] [PubMed] [Google Scholar]
- He C, Orvedahl A. Meeting Report 2007 Keystone Symposium on Autophagy in Health and Disease. Autophagy. 2007;3:527–536. doi: 10.4161/auto.4595. [DOI] [PubMed] [Google Scholar]
- Huang Q, Petros AM, Virgin HW, Fesik SW, Olejniczak ET. Solution structure of a Bcl-2 homolog from Kaposi sarcoma virus. Proc Natl Acad Sci USA. 2002;99:3428–3433. doi: 10.1073/pnas.062525799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-α regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth muscle cells. Immunol Cell Biol. 2006;84:448–454. doi: 10.1111/j.1440-1711.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Bahr BA, Baehrecke EH, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4. 2007 doi: 10.4161/auto.5338. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH2-terminal kinase. Mol Cell Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Developmental Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Capan E, Zhao Y, Zhao J, Stolz D, Watkins SC, Jin S, Lu B. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J Immunol. 2006;177:5163–5168. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- Liu J, Lin A. Role of JNK activation in apoptosis: A double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- Lu B, Capan E, Li C. Autophagy induction and autophagic cell death in effector T cells. Autophagy. 2007;3:158–159. doi: 10.4161/auto.3637. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Toumelin GL, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, et al. Functional and physical interaction between Bcl-xL and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K, Antonsson B, Magnenat E, Camps M, Muda M, Chabert C, Gillieron C, Boschert U, Vial-Knecht E, Martinou JC, Arkinstall S. Bcl-2 undergoes phosphorylation by c-Jun N-terminal kinase/stress-activated protein kinases in the presence of the constitutively active GTP-binding protein Rac1. J Biol Chem. 1997;272:25238–25242. doi: 10.1074/jbc.272.40.25238. [DOI] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. Signalling and autophagy regulation in health, aging and disease. Mol Aspects Med. 2006;27:411–425. doi: 10.1016/j.mam.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282:13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host & Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Reed JC. A day in the life of the Bcl-2 protein: does the turnover rate of Bcl-2 serve as a biological clock for cellular lifespan regulation? Leuk Res. 1996;20:109–111. doi: 10.1016/0145-2126(95)00135-2. [DOI] [PubMed] [Google Scholar]
- Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- Talloczy Z, Jiang W, Virgin HW, IV, Leib DA, Scheuner D, Kaufman RJ, Eskelinen EL, Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2α kinase signaling pathway. Proc Natl Acad Sci USA. 2002;99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Ventura JJ, Hubner A, Zhang C, Flavell RA, Shokat KM, Davis RJ. Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell. 2006;21:701–710. doi: 10.1016/j.molcel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G2/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- Zheng C, Xiang J, Hunter T, Lin A. The JNKK2-JNK1 fusion protein acts as a constitutively active c-Jun kinase that stimulates c-Jun transcription activity. J Biol Chem. 1999;274:28966–28971. doi: 10.1074/jbc.274.41.28966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.