Abstract
Cellular proliferation and tissue remodeling are central to the regenerative response after a toxic injury to the liver. To explore the role of plasminogen in hepatic tissue remodeling and regeneration, we used carbon tetrachloride to induce an acute liver injury in plasminogen-deficient (Plgo) mice and nontransgenic littermates (Plg+). On day 2 after CCl4, livers of Plg+ and Plgo mice had a similar diseased pale/lacy appearance, followed by restoration of normal appearance in Plg+ livers by day 7. In contrast, Plgo livers remained diseased for as long as 2.5 months, with a diffuse pale/lacy appearance and persistent damage to centrilobular hepatocytes. The persistent centrilobular lesions were not a consequence of impaired proliferative response in Plgo mice. Notably, fibrin deposition was a prominent feature in diseased centrilobular areas in Plgo livers for at least 30 days after injury. Nonetheless, the genetically superimposed loss of the Aα fibrinogen chain (Plgo/Fibo mice) did not correct the abnormal phenotype. These data show that plasminogen deficiency impedes the clearance of necrotic tissue from a diseased hepatic microenvironment and the subsequent reconstitution of normal liver architecture in a fashion that is unrelated to circulating fibrinogen.
The ability of the liver to regenerate after a physical or toxic injury requires both a well orchestrated proliferation of liver cells as well as tissue remodeling events to restore hepatic architecture. Although the control of the proliferative response is not fully understood, the cytokine-mediated activation of the signal transducer and activator of transcription-3 (1, 2), inducible nitric oxide synthetase (3), and CCAAT enhancer-binding protein-β (4) have been shown to play key roles in the regulation of hepatocyte proliferation and maintenance of glucose homeostasis during liver regeneration. Concomitant to these processes, the regenerating liver also requires the restructuring of the extracellular matrix (ECM) to restore the functional and lobular organization of proliferating cells. The hepatic ECM forms a dynamic scaffold for liver cells, serves as a reservoir for growth-related molecules, and undergoes degradation during liver regeneration, rendering hepatocytes responsive to mitogenic stimuli in vivo (5–8). Interestingly, both matrix modification and activation of growth factors may be facilitated by the urokinase-type plasminogen activator (uPA; ref. 9). A role of uPA in the control of liver regeneration is readily inferred from the findings that hepatocyte proliferation is transiently impaired after partial hepatectomy of uPA-deficient mice (10). Although the mechanism or mechanisms used by uPA to modulate liver regeneration are unknown, the uPA-mediated conversion of plasminogen to the active protease plasmin and the subsequent proteolytic degradation of ECM components may be biologically important in liver regeneration and remodeling (9).
The plasminogen activator/plasmin system is critical to the maintenance of hemostasis and vascular patency through degradation of fibrin and seems to be important to a variety of physiological processes, such as tissue remodeling and cell migration associated with tissue repair (11). Plasminogen-deficient mice develop to term, grow to adulthood, and are capable of reproduction (12). Nevertheless, the phenotypic consequences of plasminogen deficiency are severe and include progressive microvascular thrombosis, delayed wound healing, and reduced life expectancy (11–15). Notably, the genetic elimination of circulating fibrinogen in plasminogen-deficient mice was shown to result in a complete rescue from all of the known spontaneous phenotypic abnormalities caused by plasminogen deficiency, suggesting that fibrinolysis is the major physiological role of plasminogen (16). However, a broader functional role for plasmin outside fibrinolysis in pathological contexts or specialized settings is likely, perhaps through the direct or indirect degradation of ECM (17) and/or activation of latent growth factors (18, 19). To define the role of plasminogen in hepatic proliferative response and ECM remodeling more precisely, we studied liver repair after a toxic injury in mice genetically engineered to lack plasminogen, fibrinogen, or both. We report that the loss of plasminogen has no obvious untoward effect on hepatocyte proliferation but leads to a severe impairment in the remodeling of the hepatic lobular architecture. The abnormal repair caused by plasminogen deficiency is not rescued by genetically superimposing the loss of the fibrinogen Aα chain gene and points to a fibrinolysis-independent role of plasminogen in the liver.
Materials and Methods
Reagents and Instruments.
CCl4 was obtained from Aldrich; the sedatives ketamine and xylazine were obtained from Phoenix Pharmaceuticals (St. Joseph, MO); and acepromazine maleate was from Fort Dodge Laboratories (Fort Dodge, IA). Tissues were fixed with 10% (vol/vol) formalin from Accra Laboratory (Swedesboro, NJ). The Bio-Rad Protein Assay was purchased from Bio-Rad. Biochemical markers of liver function were determined by an automated enzymatic assay by using the Vistros Chemistry Systems 950 (Johnson & Johnson, Rochester, NY). Values are shown as means ± SD, and statistical significance was assessed by unpaired t test, with a significance level at P < 0.05.
Gene-Targeted Mice.
Mice with a targeted disruption of the genes coding for plasminogen (Plgo), fibrinogen (Fibo), or both (Plgo/Fibo) were of a mixed 129 SvJ/CF-1/NIH Black Swiss background (12, 16, 20). The genotypes of mice were established by multiplex PCR by using specific primers that identify endogenous and targeted alleles for the plasminogen and fibrinogen genes and tail or ear biopsy DNA as template (16). The initial experimental challenges included 2- to 4-month-old littermate pairs of Plgo and Plg-sufficient (Plg+) mice; similar pairing for all four genotypes (Plg+/Fib+, Plgo, Fibo, Plgo/Fibo) was also used for experiments with Plgo/Fibo mice. Mice of all genotypes were housed together in standard facilities. Animal protocols were approved by the Institutional Animal Care and Use Committee of the Children's Hospital Research Foundation.
Liver Injury.
Gene-targeted and control mice were injected intraperitoneally with 0.5 ml of CCl4 per kg of body weight as a 50% (vol/vol) solution in corn oil (21). Mice were examined daily and killed on days 2–70 after CCl4. At the time of death, mice were weighed and anesthetized intramuscularly with 0.1 ml of ketamine:xylazine:acepromazine (4:1:1) per 30 g of body weight, and blood samples were collected from the inferior vena cava. Liver samples were harvested from the same region of the liver lobes to reduce sampling variability among experimental and control mice. The extent of injury was determined in stained liver sections by using computer-assisted morphometry (National Institutes of Health image/ppc 1.56b30). For such analyses, the contour of areas of centrilobular injury was outlined completely, and the area within the drawing was used as a unit. For each liver, the cross-sectional areas within a minimum of 5 randomly selected units were analyzed without prior knowledge of the genotypes. Determination of serum albumin, alanine aminotransferase, and bilirubin was performed in plasma by using an automated enzymatic assay with the Vistros Chemistry Systems 950, and liver protein concentration was determined by the Bradford-method-based Bio-Rad assay (21). The proliferative response after CCl4 was measured by the incorporation of BrdUrd by hepatocytes in liver sections by using the Cell Proliferation Kit (Amersham Pharmacia; ref. 21). For each liver sample, the hepatocyte-labeling index (percentage of hepatocytes incorporating BrdUrd) was calculated by counting BrdUrd-labeled and unlabeled hepatocytes in 10 high-power fields (≈100 hepatocyte nuclei per field) by an investigator unaware of animal genotype.
Fibrinogen and Trichrome Stains.
Fibrinogen immunostaining in liver sections was done with a rabbit anti-mouse antiserum as described (12) and with the Vectastain ABC-AP detection system (Vector Laboratories). Fast Red TR/Naphthol AS-MX (Sigma) was used to detect alkaline phosphatase activity in situ. The standard Mason's trichrome staining procedure was used to detect connective tissue proteins (such as collagen) in liver sections of control and experimental animals.
Results
Abnormal Tissue Remodeling in Plgo Livers.
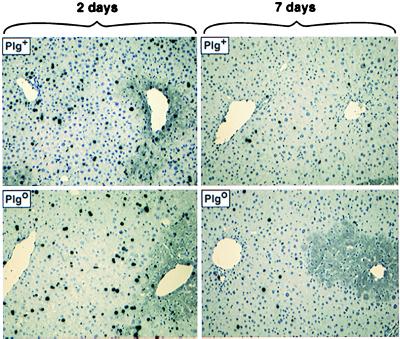
A single dose of CCl4 is known to cause an acute necroinflammatory injury to centrilobular hepatocytes, which is well established within 2 days (21). To examine the impact of plasminogen deficiency in the hepatic regenerative response, we injected CCl4 into Plgo and Plg+ mice. Survival after CCl4 was similar in mice of both genotypes. On day 2 after CCl4, Plgo livers were grossly indistinguishable from those of Plg+ littermates and had a diffuse pale/lacy appearance (Fig. 1). As expected, livers of Plg+ mice experienced a gradual restoration of normal gross appearance over a 7- to 14-day observation period. In contrast, Plgo livers remained overtly diseased on day 14, having the same diffuse pale/lacy gross appearance that had been seen on day 2 after CCl4.
Figure 1.
Persistent injury of Plgo livers after CCl4. (Upper) Plg+ livers have a diffuse pale/lacy appearance 2 days after CCl4, followed by restoration of normal appearance by days 7 and 14 (arrows point to residual areas of abnormal appearance). (Lower) In contrast, although the same diseased appearance is seen in Plgo livers on day 2, absence of plasminogen leads to a persistent diseased appearance at days 7 and 14. Although not depicted in the figure, Plgo livers on days 30 and 70 resemble those on day 14.
To examine the development and resolution of the injury at the cellular level, microscopic analyses were performed in Plgo and Plg+ livers. On day 2 after injury, liquefaction necrosis was present in centrilobular hepatocytes in both genotypes, with minimal inflammation and intact cellular components in the unaffected areas of the liver lobule (Fig. 2). Venular and sinusoidal endothelial cells, Kupffer cells, and bile duct cells appeared unaffected. Computer-based morphometric analyses of diseased areas showed that CCl4 induced a similar extent of injury in livers of both genotypes, identifying no increased susceptibility of Plgo mice to the initial toxic insult (data not shown). Systematic analysis of samples of each one of the four liver lobes on days 7, 14, 30, and 70 showed that Plg+ livers experienced a nearly complete resolution of the centrilobular injury and normalization of the lobular architecture by day 7. A few localized areas of residual centrilobular inflammation were observed within the occasional focal lesions of Plg+ livers. In contrast, the microscopic appearance of Plgo livers on days 7–30 was characterized by prominent centrilobular lesions that were indistinguishable to the lesions noted on day 2 after CCl4 (Fig. 2). By day 30, the same pattern of injury was still present, but normal-appearing hepatocytes were occasionally seen within a few injured areas, indicating that Plgo mice maintained at least some potential to repopulate the diseased areas (data not shown). The predominant finding at this time point, however, was the ongoing persistence of the centrilobular lesion seen in Plgo livers. On day 70 after CCl4, all of the centrilobular areas were still abnormal, but the eosinophilic appearance of the injured areas had been replaced by normal-appearing hepatocytes amidst basophilic amorphous granular deposits of dystrophic calcifications, a nonspecific histologic alteration encountered in areas of long-standing injury (Fig. 2). In Plg+ livers, hepatic histology was essentially normal on day 70, except for rare foci of slight dystrophic calcification (present in ≈1–2% of centrilobular regions examined).
Figure 2.
Persistent centrilobular injury of Plgo livers. Sections of Plg+ livers show an injury to centrilobular hepatocytes 2 days after CCl4, followed by normalization by day 14. In contrast, the centrilobular lesion seen in Plgo livers on day 2 persists at day 14, with no evidence of ongoing repair. At day 70, the centrilobular space of Plgo livers contains a few normal-appearing hepatocytes and abundant dystrophic calcification (Insert), which is also observed sporadically in Plg+ livers (arrows). (Magnifications: panels, ×200; Insert, ×400.)
A single injection of CCl4 would be expected to induce an acute hepatic injury but not the persistent injury observed in Plgo littermates. In animals of both genotypes, serum alanine aminotransferase levels (marker of liver cell injury) increased nearly 100-fold by day 2 after CCl4 and subsequently returned to baseline levels by day 7 in a parallel fashion (data not shown), which is consistent with an acute CCl4 injury that is short-lived, regardless of animal genotype. Despite the absence of ongoing liver injury, however, Plgo mice were unable to remove necrotic cells and repopulate the centrilobular injury with normal hepatocytes.
Plgo Livers Have Normal Proliferative Response.
To determine whether the defect observed in Plgo mice was due to an abnormal mitotic response by liver cells, we examined the proliferative response by analyzing the incorporation of BrdUrd by hepatocytes at different time points after CCl4. In Plg+ and Plgo livers, the centrilobular injury resulted in a marked increase in BrdUrd-hepatocytes when examined 2 days after the initial challenge. This increase was similar for both genotypes (Plg+ = 20.9 ± 8.6% vs. Plgo = 16.1 ± 9%; P > 0.05). After the initial rise, proliferation decreased to baseline levels (<1%) in both Plg+ and Plgo livers by day 7. In Plg+ livers, the level of proliferation seemed to decrease coordinately with the reconstitution of the centrilobular zones by normal-appearing hepatocytes. Interestingly, proliferation of hepatocytes in Plgo livers followed a similar return to baseline levels by days 7–14, despite the persistent centrilobular injury (Fig. 3). Therefore, it seemed that the normal proliferative response in Plgo livers noted 2 days after CCl4 provided these livers with an appropriate cellular mass to maintain physiologic function, as supported by normal serum albumin and bilirubin levels in Plg+ and Plgo mice by day 7 after CCl4 (data not shown).
Figure 3.
BrdUrd labeling after liver injury. BrdUrd-hepatocytes are abundant and distributed uniformly throughout the noninjured lobular region 2 days after CCl4 in Plg+ and Plgo livers. Thereafter, labeled hepatocytes are infrequently found in Plg+ livers. Note that labeled hepatocytes are similarly inconspicuous in Plgo livers, despite the persistent centrilobular injury at day 7. (Magnification: ×200.)
Based on these findings, we reasoned that if Plgo livers could mount an adequate proliferative response but were unable to effectively clear and organize damaged centrilobular zones, then these livers would increase in size after CCl4. Indeed, expressing liver weight as a percentage of body weight, Plgo livers gradually enlarged and weighed more than Plg+ livers at day 14 (8.1 ± 1.6% vs. 6 ± 0.4%; P < 0.007) and day 70 (8.1 ± 1.3% vs. 5.3 ± 0.9%; P < 0.001). Total hepatic protein was also increased in Plgo mice (Plgo = 144 ± 34 mg vs. Plg+ = 88.3 ± 8 mg; P < 0.03) at day 14, suggesting that the increase in liver size on day 14 was a consequence of the persistent deposition of cellular debris and ECM proteins in the injured centrilobular zones of Plgo animals.
Fibrin Accumulates in Diseased Plgo Livers.
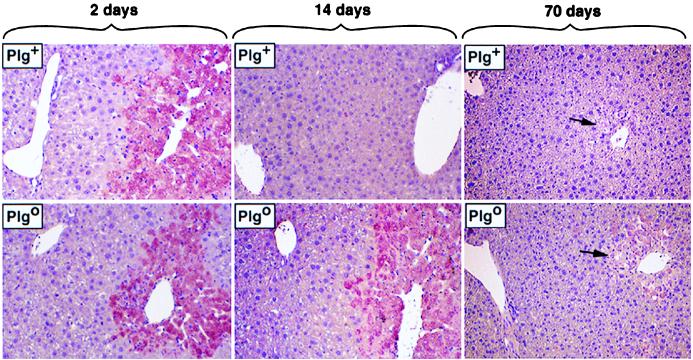
To examine the mechanisms leading to the persistent centrilobular lesion in Plgo livers, we explored whether fibrin accumulates in Plgo livers. Fibrin is an important proteolytic target for plasmin and is a consistent component of the ECM within wound fields (11). Immunohistochemical analyses of liver sections 2 days after CCl4 revealed a prominent and early deposition of fibrin within injured hepatic foci of both Plg+ and Plgo mice (Fig. 4). On day 7, fibrin deposition was no longer detected in Plg+ livers, which coincided with normalization of histology. In contrast, accumulation of fibrin or fibrin-related material was easily detected in diseased centrilobular zones of Plgo livers on day 14 (Fig. 4) and for as long as 30 days after CCl4 (data not shown). Fibrin was a component of the lobular spaces previously occupied by injured hepatocytes, and no fibrin deposits were seen in sinusoidal spaces, in the central veins, or in the nonaffected areas of the lobule. These findings are consistent with an impediment in the clearance of fibrin from diseased areas, as reported for other models of tissue injury (11, 15, 22). The persistent fibrin deposition within the injured hepatic foci of Plgo mice suggested—but did not prove—that this matrix component may form a mechanical impediment to the removal of necrotic debris and to the reconstitution of the centrilobular zones by normal hepatocytes in the absence of plasmin-mediated proteolysis.
Figure 4.
Fibrin deposition in Plgo livers. Immunohistochemical staining showing fibrin deposition in diseased areas (pink) of Plg+ and Plgo livers 2 days after CCl4. In Plg+ livers, resolution of injury is accompanied by the timely removal of fibrin. In the absence of plasminogen, fibrin deposits persist up to day 14 after toxic injury; at day 70, minimal residual immunostaining is seen in the area of dystrophic calcification (arrows). (Magnification: ×200.)
Fibrin Deficiency Does Not Rescue the Abnormal Repair of Plgo Livers.
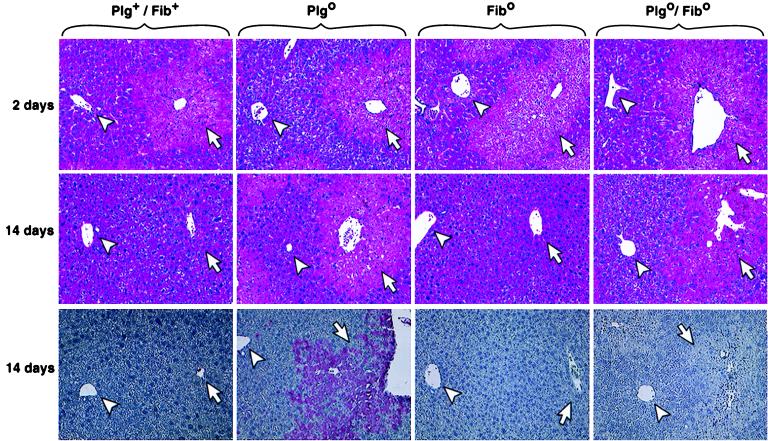
To determine the role of fibrin in these processes directly, we examined the liver repair in mice with a combined deficiency in plasminogen and fibrinogen. Plgo, Fibo, and Plgo/Fibo mice and Plg+/Fib+ littermates were challenged with CCl4 simultaneously. The livers of mice of all four genotypes had the same gross and microscopic appearance 2 days after CCl4, as described above for Plg+ and Plgo mice (data not shown). On days 7 and 14, the livers of Fibo mice regained the normal appearance, in parallel with Plg+/Fib+ littermates, suggesting that the loss of fibrin alone does not appreciably hinder this reparative process. However, the loss of circulating fibrinogen did not correct the abnormal liver repair observed in Plgo mice. Rather, Plgo/Fibo livers had a diffuse, pale/lacy gross appearance that was indistinguishable from that observed in livers of Plgo littermates (data not shown). Furthermore, the persistent liver injury in Plgo/Fibo mice was shown microscopically to be similar to that seen in Plgo livers, with the presence of unresolved centrilobular lesions on day 14 after injury (Fig. 5). The only obvious microscopic difference between the hepatic lesions of Plgo and Plgo/Fibo mice was the distinct absence of immunodetectable fibrin-related material in diseased areas of Plgo/Fibo (Fig. 5), whereas staining of ECM proteins by the Mason's trichrome method showed an intense signal in damaged livers of both Plgo and Plgo/Fibo mice (data not shown).
Figure 5.
Fibrin deficiency does not rescue the lesion of Plgo livers. Liver sections show a similar centrilobular injury regardless of the genotype 2 days after CCl4. On day 14, histology of Plg+/Fib+ and Fibo livers is normal, whereas the lesion persists in Plgo and Plgo/Fibo livers. (Bottom) Immunohistochemical staining shows the characteristic accumulation of fibrin in diseased Plgo livers 14 days after CCl4, whereas no specific staining is noted in diseased Plgo/Fibo livers. (Arrowheads, portal vein; arrows, centrilobular region; magnification: ×200.)
Discussion
These studies identify a major defect in hepatic tissue remodeling in plasminogen-deficient mice in a well established model of toxic liver injury. Neither the initial development of the hepatic injury by CCl4 nor the proliferative response was influenced by the presence of plasminogen; however, the ability of the liver to clear necrotic tissue and to repopulate and organize the diseased areas by regenerating hepatocytes was dramatically impaired by the lack of plasminogen. As a consequence, Plgo livers had areas of persistent centrilobular injury. Only several weeks after hepatic repair was complete in control animals did the livers of Plgo mice show evidence of repair and cellular repopulation of damaged centrilobular zones. The development of marked dystrophic calcifications in the injured zones led to a grossly diseased appearance and persistently abnormal hepatic architecture as long as 2.5 months after toxic injury. Despite the persistence of the diseased areas, Plgo livers had no evidence of ongoing cellular injury and had normal synthetic function, consistent with the appropriate proliferative response documented after the toxic insult. Notably, the acquisition of a nonreplicating quiescent phenotype by hepatocytes after the initial surge of DNA synthesis recapitulates the normal proliferative profile that follows CCl4 and shows that plasminogen deficiency does not impair this biological process.
The coordinate entry of the liver cells into the cell cycle is key to an adequate restoration of the cellular mass after an injury. The normal proliferative response observed in Plgo livers suggests that plasmin-mediated proteolysis is not crucial to the mitotic response during liver regeneration through modification of ECM components and/or activation of growth factors (9, 23). These findings are of particular interest in view of the recent report of delayed hepatocellular proliferation and increased foci of cellular necrosis after ≈70% partial hepatectomy in mice lacking uPA (10). The seemingly different effects of uPA and plasminogen deficiency on hepatocyte proliferation may be attributed to the use of two different models of liver injury. In partial hepatectomy, removal of two-thirds of the liver mass triggers a regenerative response in the absence of cell death, inflammation, or fibrosis, whereas CCl4 injury induces cellular proliferation in addition to all of these three biological processes (24). The basic cellular and molecular steps that take place during liver regeneration, however, are shared by both models except for differences related to the timing of the molecular response and involvement of progenitor cells (24, 25). Moreover, the presence of injured hepatocytes in models of acute injury adds a reparative dimension to the regenerative response, in which necrotic cells must be removed to allow proliferative hepatocytes to repopulate the diseased area. In this regard, studies in Plgo livers highlight the reparative dimension that takes place after a toxic injury. Without plasmin-mediated proteolysis, removal of necrotic hepatocytes is profoundly impaired and produces a persistent diseased centrilobular appearance despite an adequate proliferative response.
Although not yet directly documented, uPA- and plasminogen-deficient mice may have differences in hepatic regeneration based on either (i) the availability of tissue-type plasminogen activator-mediated activation of plasminogen in uPA-deficient mice or (ii) uPA-mediated cleavage of a biologically relevant nonplasminogen substrate. For example, uPA may stimulate liver cell proliferation directly through the activation of hepatocyte growth factor (23, 26), a property not yet established for plasminogen. However, the simultaneous enrollment of mice lacking plasminogen activators or plasminogen in liver regeneration experiments with partial hepatectomy or CCl4 will be necessary to establish definitively any independent role of these proteases in the proliferative response of the liver. To this end, applying the same model of CCl4 injury used for Plgo mice, we have observed a similar defective tissue repair in uPA-deficient mice, which parallels the findings in Plgo livers (J.A.B., unpublished work). Thus, we favor a model in which local plasmin generation is central to liver repair, and one potential mechanism by which plasmin contributes to the reparative process is in the clearance of necrotic cell debris and associated matrix components.
One matrix component associated with damaged tissues that might present a particularly strong impediment to tissue repair in the absence of plasminogen is fibrin. Consistent with this view, plasminogen-deficient mice have a profound impediment in wound repair in the skin that is associated with a decreased rate of keratinocyte migration from the wound edges (11). This impediment in tissue repair in plasminogen-deficient mice seems to be due to the absence of plasmin-mediated fibrinolysis, because the simultaneous removal of circulating fibrinogen corrects the abnormal skin healing times seen in Plgo mice (16). In contrast, genetically superimposing fibrinogen deficiency in Plgo mice does not fully correct the reparative process in CCl4-injured livers, pointing to a possible role of plasminogen outside fibrinolysis in the hepatic microenvironment. However, it should be noted that Fibo livers carry the targeted inactivation of the gene coding for the Aα fibrinogen chain (20). Although correct assembly of all three fibrinogen polypeptide chains has been shown to be essential for secretion both in vitro and in vivo, it is possible that the intracellular pool of Bβ- and γ-chains released from necrotic hepatocytes in Fibo mice presents a clearance challenge in the absence of plasmin. However, this hypothesis would require that very small quantities of intracellular fibrinogen Bβ- and γ-chains or their derivatives be sufficient to impede repair, because fibrinogen-related material was essentially undetectable in diseased areas of Fibo or Plgo/Fibo livers in sections immunostained with a polyclonal antibody that recognizes all three chains of fibrinogen. Although we cannot yet formally exclude the possibility that fibrinogen Bβ or γ chains released from necrotic hepatocytes constitutes an impediment to hepatic repair in plasminogen-deficient mice, the data suggest that plasmin may be important in hepatic tissue repair via a mechanism outside fibrin clearance. Interestingly, in addition to fibrinogen, other matrix glycoproteins such as fibronectin and tenascin are components of the provisional matrix after an acute CCl4-induced liver injury (27). Plasminogen may be involved in the in vivo clearance of these and other matrix components either directly or through the activation of procollagenase and growth factors (28). Although no data are presently available on growth factor and zymogen activation in Fibo or Plgo/Fibo livers, an impairment in either one of these processes by plasminogen deficiency could conceivably result in a reduction of the matrix-directed proteolysis and impair remodeling of the hepatic scaffold during liver regeneration.
Plasminogen-deficient mice may provide a valuable in vivo model for studies concerning the role of impaired proteolysis in the development of cirrhosis, an irreversible state of severe liver dysfunction that follows chronic, not self-limited injury. Central to cirrhosis is a disruption in the balance between production and removal of ECM proteins (29). Consistent with the proposed role of plasmin in the degradation of ECM proteins, our data show that plasminogen deficiency leads to a significant accumulation of proteins after a single toxic insult. Thus, the use of Plgo mice in models of chronic liver injury offers an unique opportunity to explore the role of impaired proteolysis in the development of cirrhosis. Because excessive accumulation of matrix elements plays an integral role in this form of abnormal liver repair, Plgo mice may be particularly advantageous in addressing whether a therapeutically targeted plasmin-mediated proteolysis can be used to correct alterations in matrix homeostasis.
Acknowledgments
This work was supported in part by F. Hoffman–La Roche, Ltd., Grant ROTRF 477924785 and National Institutes of Health Grants DK 02341 (to J.A.B.) and HL 47826 (to J.L.D.). The authors thank Ms. A. Emley for assistance with illustrations and Dr. W. Balistreri for insightful review of the manuscript.
Abbreviations
- Plg
plasminogen
- Fib
fibrinogen
- uPA
urokinase-type plasminogen activator
- ECM
extracellular matrix
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Cressman D E, Greenbaum L E, DeAngelis R A, Ciliberto G, Furth E E, Poli V, Taub R. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 2.Yamada Y, Kirillova I, Peschon J J, Fausto N. Proc Natl Acad Sci USA. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rai R M, Lee F Y, Rosen A, Yang S Q, Lin H Z, Koteish A, Liew F Y, Zaragoza C, Lowenstein C, Diehl A M. Proc Natl Acad Sci USA. 1998;95:13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenbaum L E, Li W, Cressman D E, Peng Y, Ciliberto G, Poli V, Taub R. J Clin Invest. 1998;102:996–1007. doi: 10.1172/JCI3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakowiew S B, Mead J E, Danielpour D, Wu J, Roberts A B, Fausto N. Cell Regul. 1987;2:535–548. doi: 10.1091/mbc.2.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell W E, Dempsey P J, Sitaric S, Peck A J, Coffey R J., Jr Endocrinology. 1993;133:1731–1738. doi: 10.1210/endo.133.4.8404616. [DOI] [PubMed] [Google Scholar]
- 7.Liu M L, Mars W M, Zarnegar R, Michalopoulos G K. Hepatology. 1994;19:1521–1527. [PubMed] [Google Scholar]
- 8.Kim T H, Mars W M, Stolz D B, Petersen B E, Michalopoulos G K. Hepatology. 1997;26:896–904. doi: 10.1002/hep.510260415. [DOI] [PubMed] [Google Scholar]
- 9.Michalopoulos G K, DeFrances M C. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 10.Roselli H T, Su M, Washington K, Kerins D M, Vaughan D E, Russell W E. Am J Physiol. 1998;275:G1472–G1479. doi: 10.1152/ajpgi.1998.275.6.G1472. [DOI] [PubMed] [Google Scholar]
- 11.Romer J, Bugge T H, Pyke C, Lund L R, Flick M J, Degen J L, Dano K. Nat Med. 1996;2:287–292. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- 12.Bugge T H, Flick M J, Daugherty C C, Degen J L. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, van den Oord J J, Collen D, Mulligan R C. Nature (London) 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 14.Ploplis V A, Carmeliet P, Vazirzadeh S, Van Vlaenderen I, Moons L, Plow E F, Collen D. Circulation. 1995;92:2585–2593. doi: 10.1161/01.cir.92.9.2585. [DOI] [PubMed] [Google Scholar]
- 15.Drew A F, Kaufman A H, Kombrinck K W, Danton M J, Daugherty C C, Degen J L, Bugge T H. Blood. 1998;91:1616–1624. [PubMed] [Google Scholar]
- 16.Bugge T H, Kombrinck K W, Flick M J, Daugherty C C, Danton M J, Degen J L. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z L, Strickland S. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 18.Brunner G, Gabrilove J, Rifkin D B, Wilson E L. J Cell Biol. 1991;114:1275–1283. doi: 10.1083/jcb.114.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odekon L E, Blasi F, Rifkin D B. J Cell Physiol. 1994;158:398–407. doi: 10.1002/jcp.1041580303. [DOI] [PubMed] [Google Scholar]
- 20.Suh T T, Holmback K, Jensen N J, Daugherty C C, Small K, Simon D I, Potter S, Degen J L. Genes Dev. 1995;9:2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- 21.Yazigi N A, Carrick T L, Bucuvalas J C, Schmidt C S, Balistreri W F, Bezerra J A. Transplantation. 1997;64:816–820. doi: 10.1097/00007890-199709270-00005. [DOI] [PubMed] [Google Scholar]
- 22.Busso N, Peclat V, Van Ness K, Kolodziesczyk E, Degen J, Bugge T, So A. J Clin Invest. 1998;102:41–50. doi: 10.1172/JCI2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mars W M, Zarnegar R, Michalopoulos G K. Am J Pathol. 1993;143:949–958. [PMC free article] [PubMed] [Google Scholar]
- 24.Fausto N, Webber E M. In: The Liver: Biology and Pathobiology. Arias I M, Boyer J L, Jakoby W B, Fausto N, Schachter D, editors. New York: Raven; 1994. pp. 1059–1084. [Google Scholar]
- 25.Schmiedeberg P, Biempica L, Czaja M J. J Cell Physiol. 1993;154:294–300. doi: 10.1002/jcp.1041540212. [DOI] [PubMed] [Google Scholar]
- 26.Naldini L, Vigna E, Bardelli A, Follenzi A, Galimi F, Comoglio P M. J Biol Chem. 1995;270:603–611. doi: 10.1074/jbc.270.2.603. [DOI] [PubMed] [Google Scholar]
- 27.Neubauer K, Knittel T, Armbrust T, Ramadori G. Gastroenterology. 1995;108:1124–1135. doi: 10.1016/0016-5085(95)90211-2. [DOI] [PubMed] [Google Scholar]
- 28.Vassalli J D, Sappino A P, Belin D. J Clin Invest. 1991;88:1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benyon R C, Arthur M J. J Pediatr Gastroenterol Nutr. 1998;27:75–85. doi: 10.1097/00005176-199807000-00013. [DOI] [PubMed] [Google Scholar]