Abstract
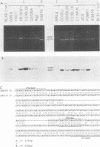
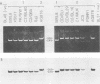
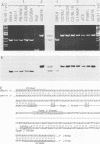
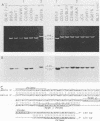
The two Epstein-Barr virus (EBV) types, EBV-1 and EBV-2, are known to differ in their EBNA-2 genes, which are 64 and 53% identical in their nucleotide and predicted amino acid sequences, respectively. Restriction endonuclease maps and serologic analyses detect few other differences between EBV-1 and EBV-2 except in the EBNA-3 gene family. We determined the DNA sequence of the AG876 EBV-2 EBNA-3 coding region and have compared it with known B95-8 EBV-1 EBNA-3 sequences to delineate the extent of divergence between EBV-1 and EBV-2 isolates in their EBNA-3 genes. The B95-8 and AG876 EBV isolates had nucleotide and amino acid identity levels of 90 and 84%, 88 and 80%, and 81 and 72% for the EBNA-3A, -3B, and -3C genes, respectively. In contrast, nucleotide sequence identity in the noncoding DNA adjacent to the B95-8 and AG876 EBNA-3 open reading frames was 96%. We used the polymerase chain reaction to demonstrate that five additional EBV-1 isolates and six additional EBV-2 isolates have the type-specific differences in their EBNA-3 genes predicted from the B95-8 or AG876 sequences. Thus, EBV-1 and EBV-2 are two distinct wild-type EBV strains that have significantly diverged at four genetic loci and have maintained type-characteristic differences at each locus. The delineation of these sequence differences between EBV-1 and EBV-2 is essential to ongoing molecular dissection of the biologic properties of EBV and of the human immune response to EBV infection. The application of these data to the delineation of epitopes recognized in the EBV-immune T-cell response is also discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adldinger H. K., Delius H., Freese U. K., Clarke J., Bornkamm G. W. A putative transforming gene of Jijoye virus differs from that of Epstein-Barr virus prototypes. Virology. 1985 Mar;141(2):221–234. doi: 10.1016/0042-6822(85)90253-3. [DOI] [PubMed] [Google Scholar]
- Arrand J. R., Young L. S., Tugwood J. D. Two families of sequences in the small RNA-encoding region of Epstein-Barr virus (EBV) correlate with EBV types A and B. J Virol. 1989 Feb;63(2):983–986. doi: 10.1128/jvi.63.2.983-986.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Zimber U., Hudewentz J., Epstein M. A. Comparison of Epstein-Barr virus strains of different origin by analysis of the viral DNAs. J Virol. 1980 Sep;35(3):603–618. doi: 10.1128/jvi.35.3.603-618.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm G. W., Hudewentz J., Freese U. K., Zimber U. Deletion of the nontransforming Epstein-Barr virus strain P3HR-1 causes fusion of the large internal repeat to the DSL region. J Virol. 1982 Sep;43(3):952–968. doi: 10.1128/jvi.43.3.952-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows S. R., Misko I. S., Sculley T. B., Schmidt C., Moss D. J. An Epstein-Barr virus-specific cytotoxic T-cell epitope present on A- and B-type transformants. J Virol. 1990 Aug;64(8):3974–3976. doi: 10.1128/jvi.64.8.3974-3976.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows S. R., Sculley T. B., Misko I. S., Schmidt C., Moss D. J. An Epstein-Barr virus-specific cytotoxic T cell epitope in EBV nuclear antigen 3 (EBNA 3). J Exp Med. 1990 Jan 1;171(1):345–349. doi: 10.1084/jem.171.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Lewis J. P., Abildgaard C. F. Prevalence of oropharyngeal excreters of leukocyte-transforming agents among a human population. N Engl J Med. 1973 Dec 20;289(25):1325–1329. doi: 10.1056/NEJM197312202892501. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Draper K. G., Kelly T. J., Wagner E. K. An unusual spliced herpes simplex virus type 1 transcript with sequence homology to Epstein-Barr virus DNA. J Virol. 1985 May;54(2):317–328. doi: 10.1128/jvi.54.2.317-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Smith G. L., Bell S. E., Hart H., Brown C., Bankier A. T., Tomlinson P., Barrell B. G., Minson T. C. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J Virol. 1988 Apr;62(4):1416–1422. doi: 10.1128/jvi.62.4.1416-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Hennessy K., Chamnankit L., Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Raab-Traub N., Heller M., Beisel C., Hummel M., Cheung A., Fennewald S., King W., Kieff E. Variations among isolates of Epstein-Barr virus. Ann N Y Acad Sci. 1980;354:309–325. doi: 10.1111/j.1749-6632.1980.tb27974.x. [DOI] [PubMed] [Google Scholar]
- Dambaugh T., Wang F., Hennessy K., Woodland E., Rickinson A., Kieff E. Expression of the Epstein-Barr virus nuclear protein 2 in rodent cells. J Virol. 1986 Aug;59(2):453–462. doi: 10.1128/jvi.59.2.453-462.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Taylor P. Genetic relations between varicella-zoster virus and Epstein-Barr virus. J Gen Virol. 1987 Apr;68(Pt 4):1067–1079. doi: 10.1099/0022-1317-68-4-1067. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Luka J., Armstrong M. E., Keller P. M., Ellis R. W., Pearson G. R. Identification of an Epstein-Barr virus glycoprotein which is antigenically homologous to the varicella-zoster virus glycoprotein II and the herpes simplex virus glycoprotein B. Virology. 1987 Apr;157(2):552–555. doi: 10.1016/0042-6822(87)90300-x. [DOI] [PubMed] [Google Scholar]
- Gerber P., Lucas S., Nonoyama M., Perlin E., Goldstein L. I. Oral excretion of Epstein-Barr virus by healthy subjects and patients with infectious mononucleosis. Lancet. 1972 Nov 11;2(7785):988–989. doi: 10.1016/s0140-6736(72)92402-6. [DOI] [PubMed] [Google Scholar]
- Given D., Kieff E. DNA of Epstein-Barr virus. IV. Linkage map of restriction enzyme fragments of the B95-8 and W91 strains of Epstein-Barr Virus. J Virol. 1978 Nov;28(2):524–542. doi: 10.1128/jvi.28.2.524-542.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., Ooka T., Matsuo T., Kieff E. Epstein-Barr virus glycoprotein homologous to herpes simplex virus gB. J Virol. 1987 Feb;61(2):499–508. doi: 10.1128/jvi.61.2.499-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfull G., Bankier A. T., Barrell B. G., Farrell P. J. Sequence analysis of Raji Epstein-Barr virus DNA. Virology. 1988 Jun;164(2):334–340. doi: 10.1016/0042-6822(88)90546-6. [DOI] [PubMed] [Google Scholar]
- Heller M., Dambaugh T., Kieff E. Epstein-Barr virus DNA. IX. Variation among viral DNAs from producer and nonproducer infected cells. J Virol. 1981 May;38(2):632–648. doi: 10.1128/jvi.38.2.632-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., Henderson A., Kieff E. Repeat array in Epstein-Barr virus DNA is related to cell DNA sequences interspersed on human chromosomes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5916–5920. doi: 10.1073/pnas.79.19.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Wang F., Bushman E. W., Kieff E. Definitive identification of a member of the Epstein-Barr virus nuclear protein 3 family. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5693–5697. doi: 10.1073/pnas.83.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Hayward S. D. Organization of the Epstein-Barr virus DNA molecule. III. Location of the P3HR-1 deletion junction and characterization of the NotI repeat units that form part of the template for an abundant 12-O-tetradecanoylphorbol-13-acetate-induced mRNA transcript. J Virol. 1983 Oct;48(1):135–148. doi: 10.1128/jvi.48.1.135-148.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson H. B., Farrell P. J., Miller G. Sequences of the Epstein-Barr Virus (EBV) large internal repeat form the center of a 16-kilobase-pair palindrome of EBV (P3HR-1) heterogeneous DNA. J Virol. 1987 May;61(5):1495–1506. doi: 10.1128/jvi.61.5.1495-1506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson H. B., Miller G. Polymorphisms of the region of the Epstein-Barr virus genome which disrupts latency. Virology. 1988 Aug;165(2):549–564. doi: 10.1016/0042-6822(88)90599-5. [DOI] [PubMed] [Google Scholar]
- Joab I., Rowe D. T., Bodescot M., Nicolas J. C., Farrell P. J., Perricaudet M. Mapping of the gene coding for Epstein-Barr virus-determined nuclear antigen EBNA3 and its transient overexpression in a human cell line by using an adenovirus expression vector. J Virol. 1987 Oct;61(10):3340–3344. doi: 10.1128/jvi.61.10.3340-3344.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. D., Foster L., Sheedy T., Griffin B. E. The EB virus genome in Daudi Burkitt's lymphoma cells has a deletion similar to that observed in a non-transforming strain (P3HR-1) of the virus. EMBO J. 1984 Apr;3(4):813–821. doi: 10.1002/j.1460-2075.1984.tb01890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdiles B., Walls D., Triki H., Perricaudet M., Joab I. cDNA cloning and transient expression of the Epstein-Barr virus-determined nuclear antigen EBNA3B in human cells and identification of novel transcripts from its coding region. J Virol. 1990 Apr;64(4):1812–1816. doi: 10.1128/jvi.64.4.1812-1816.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W., Dambaugh T., Heller M., Dowling J., Kieff E. Epstein-Barr virus DNA XII. A variable region of the Epstein-Barr virus genome is included in the P3HR-1 deletion. J Virol. 1982 Sep;43(3):979–986. doi: 10.1128/jvi.43.3.979-986.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Large-scale rearrangement of homologous regions in the genomes of HCMV and EBV. Virology. 1987 Apr;157(2):397–413. doi: 10.1016/0042-6822(87)90282-0. [DOI] [PubMed] [Google Scholar]
- Littler E., Halliburton I. W., Powell K. L., Snowden B. W., Arrand J. R. Immunological conservation between Epstein-Barr virus and herpes simplex virus. J Gen Virol. 1988 Aug;69(Pt 8):2021–2031. doi: 10.1099/0022-1317-69-8-2021. [DOI] [PubMed] [Google Scholar]
- Littler E., Zeuthen J., McBride A. A., Trøst Sørensen E., Powell K. L., Walsh-Arrand J. E., Arrand J. R. Identification of an Epstein-Barr virus-coded thymidine kinase. EMBO J. 1986 Aug;5(8):1959–1966. doi: 10.1002/j.1460-2075.1986.tb04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Davison A. J. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 1986 May 27;14(10):4281–4292. doi: 10.1093/nar/14.10.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Frame M. C. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the exonuclease gene and neighbouring genes. Nucleic Acids Res. 1986 Apr 25;14(8):3435–3448. doi: 10.1093/nar/14.8.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Niederman J. C., Andrews L. L. Prolonged oropharyngeal excretion of Epstein-Barr virus after infectious mononucleosis. N Engl J Med. 1973 Feb 1;288(5):229–232. doi: 10.1056/NEJM197302012880503. [DOI] [PubMed] [Google Scholar]
- Morgan D. G., Niederman J. C., Miller G., Smith H. W., Dowaliby J. M. Site of Epstein-Barr virus replication in the oropharynx. Lancet. 1979 Dec 1;2(8153):1154–1157. doi: 10.1016/s0140-6736(79)92384-5. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Kurilla M. G., Griffin H. M., Brooks J. M., Mackett M., Arrand J. R., Rowe M., Burrows S. R., Moss D. J., Kieff E. Human cytotoxic T-cell responses against Epstein-Barr virus nuclear antigens demonstrated by using recombinant vaccinia viruses. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2906–2910. doi: 10.1073/pnas.87.8.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Biggin M. D., Barrell B., Roizman B. Epstein-Barr virus genome may encode a protein showing significant amino acid and predicted secondary structure homology with glycoprotein B of herpes simplex virus 1. J Virol. 1985 Dec;56(3):807–813. doi: 10.1128/jvi.56.3.807-813.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., Kieff E. A sixth Epstein-Barr virus nuclear protein (EBNA3B) is expressed in latently infected growth-transformed lymphocytes. J Virol. 1988 Jun;62(6):2173–2178. doi: 10.1128/jvi.62.6.2173-2178.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., Sample J., Wang F., Kieff E. A fifth Epstein-Barr virus nuclear protein (EBNA3C) is expressed in latently infected growth-transformed lymphocytes. J Virol. 1988 Apr;62(4):1330–1338. doi: 10.1128/jvi.62.4.1330-1338.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack A., Delius H., Zimber U., Bornkamm G. W. Two deletions in the Epstein-Barr virus genome of the Burkitt lymphoma nonproducer line Raji. Virology. 1984 Feb;133(1):146–157. doi: 10.1016/0042-6822(84)90433-1. [DOI] [PubMed] [Google Scholar]
- Rabson M., Gradoville L., Heston L., Miller G. Non-immortalizing P3J-HR-1 Epstein-Barr virus: a deletion mutant of its transforming parent, Jijoye. J Virol. 1982 Dec;44(3):834–844. doi: 10.1128/jvi.44.3.834-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A. B., Young L. S., Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J Virol. 1987 May;61(5):1310–1317. doi: 10.1128/jvi.61.5.1310-1317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. T., Rowe M., Evan G. I., Wallace L. E., Farrell P. J., Rickinson A. B. Restricted expression of EBV latent genes and T-lymphocyte-detected membrane antigen in Burkitt's lymphoma cells. EMBO J. 1986 Oct;5(10):2599–2607. doi: 10.1002/j.1460-2075.1986.tb04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Cadwallader K., Petti L., Kieff E., Rickinson A. B. Distinction between Epstein-Barr virus type A (EBNA 2A) and type B (EBNA 2B) isolates extends to the EBNA 3 family of nuclear proteins. J Virol. 1989 Mar;63(3):1031–1039. doi: 10.1128/jvi.63.3.1031-1039.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculley T. B., Sculley D. G., Pope J. H., Bornkamm G. W., Lenoir G. M., Rickinson A. B. Epstein-Barr virus nuclear antigens 1 and 2 in Burkitt lymphoma cell lines containing either 'A'- or 'B'-type virus. Intervirology. 1988;29(2):77–85. doi: 10.1159/000150032. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Yamaki M., Sakuma S., Ono Y., Takada K. Three Epstein-Barr virus (EBV)-determined nuclear antigens induced by the BamHI E region of EBV DNA. Int J Cancer. 1988 May 15;41(5):744–751. doi: 10.1002/ijc.2910410518. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Nedrud J. G., Raab-Traub N., Hanes R. A., Pagano J. S. Epstein-Barr virus replication in oropharyngeal epithelial cells. N Engl J Med. 1984 May 10;310(19):1225–1230. doi: 10.1056/NEJM198405103101905. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Shirley P., Chesney P. J., Buntin D. M., Resnick L. Detection of a second widespread strain of Epstein-Barr virus. Lancet. 1989 Sep 30;2(8666):761–765. doi: 10.1016/s0140-6736(89)90829-5. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Vesterinen E. H., Nedrud J. G., Raab-Traub N., Walton L. A., Pagano J. S. Replication of Epstein-Barr virus in human epithelial cells infected in vitro. Nature. 1983 Dec 1;306(5942):480–483. doi: 10.1038/306480a0. [DOI] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C., Sample C., Rowe M., Liebowitz D., Murray R., Rickinson A., Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990 May;64(5):2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q. Y., Rickinson A. B., Epstein M. A. A re-examination of the Epstein-Barr virus carrier state in healthy seropositive individuals. Int J Cancer. 1985 Jan 15;35(1):35–42. doi: 10.1002/ijc.2910350107. [DOI] [PubMed] [Google Scholar]
- Young L. S., Clark D., Sixbey J. W., Rickinson A. B. Epstein-Barr virus receptors on human pharyngeal epithelia. Lancet. 1986 Feb 1;1(8475):240–242. doi: 10.1016/s0140-6736(86)90776-2. [DOI] [PubMed] [Google Scholar]
- Young L. S., Dawson C. W., Clark D., Rupani H., Busson P., Tursz T., Johnson A., Rickinson A. B. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol. 1988 May;69(Pt 5):1051–1065. doi: 10.1099/0022-1317-69-5-1051. [DOI] [PubMed] [Google Scholar]
- Young L. S., Yao Q. Y., Rooney C. M., Sculley T. B., Moss D. J., Rupani H., Laux G., Bornkamm G. W., Rickinson A. B. New type B isolates of Epstein-Barr virus from Burkitt's lymphoma and from normal individuals in endemic areas. J Gen Virol. 1987 Nov;68(Pt 11):2853–2862. doi: 10.1099/0022-1317-68-11-2853. [DOI] [PubMed] [Google Scholar]
- Zimber U., Adldinger H. K., Lenoir G. M., Vuillaume M., Knebel-Doeberitz M. V., Laux G., Desgranges C., Wittmann P., Freese U. K., Schneider U. Geographical prevalence of two types of Epstein-Barr virus. Virology. 1986 Oct 15;154(1):56–66. doi: 10.1016/0042-6822(86)90429-0. [DOI] [PubMed] [Google Scholar]