Abstract
Depression is a risk factor for cardiovascular disease (CVD) perhaps mediated by hypothalamic-pituitary-adrenal (HPA) axis or vagal dysregulation. We investigated circadian mood variation and HPA-axis and autonomic function in older (≥55 years) depressed and nondepressed volunteers at risk for CVD by assessing diurnal positive and negative affect (PA, NA), cortisol, and cardiopulmonary variables in 46 moderately depressed and 19 nondepressed volunteers with elevated CVD risk. Participants sat quietly for 5-min periods (10:00, 12:00, 14:00, 17:00, 19:00, 21:00), and then completed an electronic diary assessing PA and NA. Traditional and respiration-controlled heart rate variability (HRV) variables were computed for these periods as an index of vagal activity. Salivary cortisols were collected at waking, waking+30 min, 12:00, 17:00, and 21:00 hours. Cortisol peaked in the early morning after waking, and gradually declined over the day, but did not differ between groups. PA was lower and NA was higher in the depressed group throughout the day. HRV did not differ between groups. Negative emotions were inversely related to respiratory sinus arrhythmia in nondepressed participants. We conclude that moderately depressed patients do not show abnormal HPA-axis function. Diurnal PA and NA distinguish depressed from nondepressed patients at risk for CVD, while measures of vagal regulation, even when controlled for physical activity and respiratory confounds, do not. Diurnal mood variations of older individuals at risk for CVD differ from those reported for other groups and daily fluctuations in NA are not related to cardiac autonomic control in depressed individuals.
Keywords: Cardiovascular Disease, Cortisol, Depression, Heart Rate Variability
Depression has a major impact on mortality, morbidity, and functional recovery in patients with cardiovascular disease (CVD) (Carney, Freedland, & Sheps, 2004). Several mechanisms have been proposed to explain how depression might increase CVD risk, including hypothalamic-pituitary-adrenal (HPA) axis and autonomic cardiovascular dysregulation (Musselman, Evans, & Nemeroff, 1998). Abnormal HPA-axis function may contribute to CVD risk through a variety of related risk factors, including hypertension, high lipids, insulin resistance, and abdominal obesity, together constituting the metabolic syndrome (Brown, Varghese, & McEwen, 2004). Several studies have reported basal hypercortisolism (e.g., Gotthardt et al., 1995; Plotsky, Owens, & Nemeroff, 1998), hypercortisolism in response to stress (for a review, see Burke, Davis, Otte, & Mohr, 2005), or elevated cortisol levels after awakening in depression (e.g., Bhagwagar, Hafizi, & Cowen, 2005). A variety of studies have suggested that depressed subjects have less heart rate variability (HRV) than nondepressed controls (e.g., Agelink, Boz, Ullrich, & Andrich, 2002; Carney et al., 2005; Carney et al., 2001), even when controlled for potential confounds such as age, sex, and smoking, suggesting autonomic dysregulation.
So far, little attention has been paid to circadian fluctuation of HRV and its relationship to mood variation. There is some indication that HRV is inversely associated with state negative affect (NA), stress, anxiety, and anger (e.g., Dishman et al., 2000; Schwarz, Schachinger, Adler, & Goetz, 2003). Two studies have employed ambulatory recordings to investigate the effects of mental stress throughout the day on cardiac autonomic control in healthy subjects; one reported a positive relationship between HRV and affect (Bacon et al., 2004), the other found no association (Sloan et al., 1994). Similar to HRV, little information is available on circadian mood variation in older patients at risk for CVD with or without depression. For healthy students, positive affect (PA) rises in the morning, peaks in the early afternoon, and then declines. NA may not vary across the day or may increase in the evening (Clark, Watson, & Leeka, 1989; Murray, Allen, & Trinder, 2002; Watson, Wiese, Vaidya, & Tellegen, 1999) and the circadian pattern may differ among depression subtypes. For instance, Rusting and Larsen (1998) found that severe depression is associated with a morning-worse pattern, while milder depression is associated with an evening-worse pattern.
To date, no study has investigated temporal variation in HRV, cortisol, and mood across the day and focused on differences between depressed and nondepressed individuals at risk for CVD. Recent results from Steptoe and colleagues (Steptoe, Wardle, & Marmot, 2005; Strike, Wardle, & Steptoe, 2004) demonstrate the importance of such data. The authors found a relationship between inflammatory stimulation and negative mood, while temporary PA was inversely related to cortisol and heart rate (HR).
We recruited depressed and nondepressed volunteers at risk for CVD for a risk factor study at Stanford University. Participants underwent a physical exam, ambulatory monitoring, and a laboratory stressor. Results of the stress test have been reported elsewhere (Taylor et al., 2006). In brief, the depressed patients had significantly higher C-reactive protein levels at baseline, showed significantly lower cortisol levels and respiratory sinus arrhythmia (RSA) during the stress test, and less cortisol response to stress. This paper examines whether cardiopulmonary parameters, cortisol, and mood differ across the day in the depressed and nondepressed individuals at risk for CVD. We hypothesized that PA throughout the day will be lower in depressed than nondepressed subjects, and that NA will be higher. Extrapolating from laboratory studies, we expected that HRV – and specifically RSA – would be less in depressed participants than in their nondepressed counterparts. Furthermore, we expected elevated NA and reduced PA to correspond to low RSA, and low NA and high PA to correspond to high RSA in the nondepressed group. We hypothesized the same relationship in depressed participants.
Methods
Participants
Depressed and nondepressed volunteers, age 55 or greater, at risk for CVD were recruited. To be considered high risk, subjects had to have currently or a history of hypertension and/or hypercholesterolemia. Subjects were excluded if they smoked, had had a stroke or myocardial infaction, or were female and not postmenopausal. For the depressed group, participants had to be diagnosed with DSM-IV Major Depressive Disorder in the Depression Interview and Structured Hamilton (DISH) (Freedland et al., 2002) and had to score higher than 10 in the Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961). For the control group, participants could neither be depressed or bipolar nor exceed a score of 10 on the BDI.
Of the 381 people who made inquiries, 2 could not be reached, 108 decided not to participate before the screening, 121 were interested but not eligible, 28 decided not to participate after the screening, 12 did not want to continue in the study after the interview, and 28 did not meet eligibility criteria. Forty-eight depressed and 20 nondepressed participants were studied, but, due to technical difficulties, only 46 depressed and 19 nondepressed participants were analyzed.
Demographic and medical characteristics by group and gender are listed in Table 1. There were more females than males in the depressed group and more males than females in the nondepressed group, although this difference was not statistically significant. There were no significant differences between groups in age, body mass index, or ethnicity, but the groups differed in race. In the male depressed group, one participant was African-American and one of Asian descent. In the nondepressed women, there were three African-Americans and one participant of Asian descent. All participants were at risk for CVD, and did not differ in percent hypertension or usage of lipid lowering or anti-hypertensive drugs. Four depressed females had secondary diagnoses (2, social anxiety disorder; 1, panic disorder; 1, both) while two depressed males were diagnosed with panic disorder. None of the participants in the control group had a psychiatric diagnosis. In the depressed group, 5 females and 4 males were taking selective serotonin reuptake inhibitors (SSRIs), 6 females and 2 males were taking antidepressants other than SSRIs. One male was taking SSRIs plus other antidepressants. In the nondepressed group, one female and one male were taking SSRIs.
Table 1.
Demographic and medical characteristics by group
| Depressed Females | Depressed Males | Nondepressed Females | Nondepressed Males | F or χ2abc | |
|---|---|---|---|---|---|
| N | 31 | 15 | 8 | 11 | χ2 = 3.58 |
| age (years) | 62.8 (6.9) | 61.1 (5.6) | 61.1 (5.6) | 64.3 (6.0) | F = 0.18; F = 0.15; F = 1.93 |
| Race (% Caucasian) | 100 | 86.7 | 50 | 100 | χ2 = 20.44*** |
| Ethnicity (% Hispanic or Latino) | 0 | 6.7 | 12.5 | 9.1 | χ2 = 3.27 |
| BMI | 29.6 (6.4) | 29.3 (6.4) | 27.7 (4.4) | 27.6 (3.7) | F = 1.2; F = 0.13; F = 0.01 |
| Hypercholesterolemic d(%) | 35.5 | 6.7 | 12.5 | 0 | χ2 = 9.34* |
| Taking lipid lowering medication (%) | 16.1 | 26.7 | 12.5 | 18.2 | χ2 = 0.97 |
| Hypertensive e(%) | 51.6 | 80.0 | 62.5 | 72.7 | χ2 = 4.04 |
| Taking anti-hypertensive medication (%) | 48.4 | 53.3 | 37.5 | 63.6 | χ2 = 1.4 |
| Taking antidepressant medication (%) | 35.5 | 46.7 | 12.5 | 9.1 | χ2 = 5.78 |
| BDI | 25.35 (8.85) | 24.64 (9.83) | 2.63 (2.45) | 5.36 (3.8) | F = 87.45 ***; F = 0.2 ; F = 0.59 |
| HRSD | 18.19 (5.53) | 16.93 (5.71) | 5.14 (13.61) | 2 (2.1) | F = 55.85 ***; F = 1.38; F = 0.25 |
| PANAS - PA | 23.17 (6.37) | 25.53 (8.37) | 39.14 (4.91) | 34.45 (4.97) | F = 42.95 ***; F = 0.37; F = 3.45 |
| PANAS - NA | 27.57 (9) | 27.13 (8.21) | 14.86 (5.18) | 14.09 (2.27) | F = 32.92 ***; F = 0.07; F = 0.01 |
| PSS | 24.23 (3.15) | 23.6 (2.06) | 20.38 (2.2) | 19.73 (3) | F = 24.1 ***; F = 0.66; F = 0.99 |
Note. Values are expressed as number, mean (SD), or percent; BMI = Body Mass Index; BDI = Beck Depression Inventory; HRSD = Hamilton Rating Scale for Depression; PANAS = Positive and Negative Affect Schedule; PA = positive affect; NA = negative affect; PSS = Perceived Stress Scale.
F ratios from two way (group × gender) analyses of variance (ANOVAs) (df = 1, 64) (group, gender, group × gender); χ2 (df = 1) from contingency tables.
* = p < 0.05.
** = p < 0.001.
Total cholesterol > 240.
SBP > 140 and / or DBP > 90.
Measurements
Ambulatory cardiopulmonary data and diary entries were recorded with the LifeShirt System (Vivometrics, Ventura, CA). The device measures continuous HR, respiration, and accelerometry (for details, see Wilhelm, Roth, & Sackner, 2003). Cortisol samples were collected with the Medication Event Monitoring System (MEMS; AARDEX Ltd., Zug, Switzerland), enabling us to check whether samples were collected at the appropriate times. Problems with adherence were minimal.
Psychological Measures
Baseline questionnaires
Baseline questionnaires included the BDI, the Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960), the Positive and Negative Affect Schedule (PANAS) (Watson, Clark, & Tellegen, 1988), and the Perceived Stress Scale (PSS) (Cohen, Kamarck, & Mermelstein, 1983).
Experimental questionnaires
Participants completed a mood questionnaire on the hand-held computer of the LifeShirt at 6 times a day, at 10:00, 12:00, 14:00, 17:00, 19:00, and 21:00 hours. The questionnaire was a short form of the PANAS with five items each from the PA (happy, interested, enthusiastic, alert, determined) and NA (irritable, distressed, hostile, ashamed, nervous) scale.
Physiological measures
Cardiac measures
A single lead ECG was collected with a sampling rate of 200 Hz.
Respiratory measures
The LifeShirt garment incorporates plethysmographic sensors sewn into the shirt at the level of the rib cage and abdomen. The sensors transduce breath waveforms with a sample rate of 50 Hz, from which pulmonary variables can be derived.
Activity measures
An accelerometer is located on the anterior surface at the level of the rib cage in the LifeShirt system. The accelerometer output is a summation of two axes of motion, and was sampled at 10 Hz.
Cortisol
Saliva was collected using cotton swabs in ‘Salivette’ devices (Sarstedt, Inc., Newton, NC). Salivary cortisol was assayed using luminescence immunoassay (LIA) reagents provided by Immuno-Biological Laboratories, Inc. Hamburg, Germany. Assay sensitivity was 0.015 µg/dl.
Procedures
Stanford University and VA Palo Alto Institutional Review Boards granted study approval and participants provided informed consent. Participants completed questionnaires and interviews before being scheduled for the physical exam, the ambulatory monitoring, and a psychological stress test on 3 consecutive weekdays. Subjects received $50 for their participation in the study. Data from the ambulatory monitoring are reported.
Participants were instructed to obtain saliva samples at the time of waking, 30 min later, and then at 12:00, 17:00 and 21:00 hours. Participants collected saliva using cotton swabs and refrigerated each sample immediately after collection. Participants were not to eat, drink, smoke, brush their teeth, or use mouthwash in the 30 min before collection and not to drink alcohol during the 8–10 hours prior to collecting samples or during the days of collection. Participants were scheduled to arrive at 09:00 on the day of ambulatory monitoring to be outfitted with a LifeShirt. After starting the physiologic recording, subjects completed a respiration calibration procedure for the calculation of tidal volume. Subjects were given a Timex watch (Timex, North Little Rock, AR) that was programmed to sound at 10:00, 12:00, 14:00, 17:00, 19:00 and 21:00 hours to remind them to fill out mood questionnaires and to collect saliva at 3 of those times. Subjects were told to sit quietly with their eyes open for 5 min before completing each survey. Subjects completed the first quiet time and the first survey for the 10:00 assessment in the experimenter’s office. If no further questions arose, subjects left and continued with their regular daily activities while completing the surveys at the designated times.
Data reduction
Psychological measures
PA and NA scores for each subject were computed by averaging the scores of the five items of each subscale.
Physiological measures
Cardiac measures
Only data from the instructed 5-min quiet sitting periods were analyzed since ambulatory physical activity has a profound influence on HR and RSA and can potentially bias such data (Grossman, Wilhelm, & Spoerle, 2004). For each 5-min segment, five 1-min averages of HR extracted by the VivoLogic software were averaged. For the HRV measures, the ECG and respiration signals for each of the 5-min segments were imported into customized programs (for details, see Giese-Davis et al., 2006) and edited for artifacts. The variance in the RR-interval signal was decomposed via spectral analysis to yield a set of frequency domain indices representing the spectral power within distinct frequency bands [HF (0.13 – 0.5 Hz), LF (0.04 – 0.13 Hz), and VLF (0.003 – 0.04 Hz)]. The parameters were transformed by natural logarithm to produce an approximately normal distribution. Since variations in respiration can bias the relationship between traditional spectral measures and vagal activity (Grossman & Taylor, 2007), transfer-function RSA (TF-RSA) was quantified as the magnitude of the transfer function relating RR-interval oscillations to tidal volume oscillations at the peak respiratory frequency (Saul et al., 1991). TF-RSA data were excluded if spectral coherence between tidal volume and RR-interval was below 0.5 (Rottenberg, Wilhelm, Gross, & Gotlib, 2002), because less coherence would indicate sources for RR-interval variation other than respiration.
Respiratory measures
A fixed volume least square calibration (e.g., Stromberg, Dahlback, & Gustafsson, 1993) was applied in order to assign absolute tidal volume values to the breathing curves. 1-min averages for respiratory variables were extracted and averaged over each 5-min segment.
Activity measures
Segment specific means of the accelerometer values were generated by averaging the 1-min averages over 5 min. 1-min segments with physical activity levels untypical for quiet sitting or respiratory waveforms that indicated speaking were excluded for those calculations.
Statistical analyses
First, the groups were compared on demographic, clinical, and control variables. Continuous measures were evaluated with Analyses of Variance (ANOVAs) with the factors group (depressed vs. nondepressed) and gender, while differences in categorical variables were tested with χ2 tests.
Following the recommendation by Bagiella and colleagues (Bagiella, Sloan, & Heitjan, 2000) for analyzing psychophysiological data in repeated-measures experiments, mixed-effects models assuming autoregressive covariance with the factors group (depressed vs. nondepressed), gender, and time (10:00, 12:00, 14:00, 17:00, 19:00, 21:00 for psychophysiological data other than cortisol, and waking, waking + 30, 12:00, 17:00, 21:00 for cortisol) were used to examine potential main effects or interactions in any of the psychological or physiological variables during the ambulatory recording. To decrease the potential for alpha inflation, a small number of variables (PA, NA, TF-RSA, HF, LF, VLF) were assigned as primary measures, while all other variables were explored as secondary measures. The relationships between diary entries and HRV were examined with random coefficient models assuming unstructured covariance. Separate models were computed for each combination of affect (PA, NA) and HRV (VLF, LF, HF, TF-RSA) variables. To avoid misleading parameters and multicollinearity problems in regression (Kraemer & Blasey, 2004), diary ratings were first centered and then entered in their continuous form as independent variables. HRV variables were dependent variables in the model. A 5% two-tailed significance level was used for all analyses except for secondary measures, for which probability was set at p < .01.
Results
Baseline differences
At the initial assessment, the depressed groups showed significantly higher scores on the depression inventories than the control groups (see Table 1). In addition, depressed participants rated themselves higher on the PSS than the controls, and reported lower PA and higher NA on the PANAS.
Control measures
Cortisol data was missing for 2% of all time points. Mood data were missing for 9% of all segments and physiological data were missing for 5% of all segments. Reasons for data loss were non-compliance or technical difficulties. Accelerometer data could not be retrieved for eight subjects (four depressed women, two depressed men, one nondepressed woman, and one nondepressed man). A mixed effects model analysis of activity did not indicate main effects for group, gender, or time, but significant interaction effects for group × gender and gender × time (see Table 2).
Table 2.
Results of mixed effects models for endocrine, affective, and cardiopulmonary variables
| Group (G) | Gender (GE) | Time (T) | G × GE | G × T | GE × T | G × GE × T | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| Control measures | ||||||||||||||
| ACCM | 1.74 | n.s. | 1.74 | n.s. | 1.00 | n.s. | 7.46 | .008 | 0.56 | n.s. | 2.63 | .024 | 0.27 | n.s. |
| Coherence | 0.43 | n.s. | 1.48 | n.s. | 0.79 | n.s. | 0.26 | n.s. | 0.63 | n.s. | 0.16 | n.s. | 1.10 | n.s. |
| RR SD(mean) | 0.28 | n.s. | 5.05 | .027 | 1.46 | n.s. | 0.16 | n.s. | 0.32 | n.s. | 0.55 | n.s. | 0.31 | n.s. |
| RR SD(SD) | 0.16 | n.s. | 2.11 | n.s. | 1.98 | n.s. | 0.10 | n.s. | 2.00 | n.s. | 0.71 | n.s. | 0.45 | n.s. |
| Primary outcome measures | ||||||||||||||
| NA | 23.26 | < .001 | 0.09 | n.s. | 0.70 | n.s. | 1.69 | n.s. | 0.58 | n.s. | 0.08 | n.s. | 0.08 | n.s. |
| PA | 13.71 | < .001 | 0.14 | n.s. | 1.97 | n.s. | 4.10 | .046 | 1.68 | n.s. | 1.02 | n.s. | 1.30 | n.s. |
| Log Cortisol | 2.79 | n.s. | 3.90 | n.s. | 71.08 | < .001 | 0.03 | n.s. | 0.85 | n.s. | 2.02 | n.s. | 1.74 | n.s. |
| ln VLF | 0.04 | n.s. | 9.72 | .003 | 2.40 | .038 | 2.17 | n.s. | 0.65 | n.s. | 1.03 | n.s. | 0.72 | n.s. |
| ln LF | 0.40 | n.s. | 3.07 | n.s. | 2.61 | .025 | 0.69 | n.s. | 1.63 | n.s. | 1.81 | n.s. | 0.62 | n.s. |
| ln HF | 0.01 | n.s. | 0.87 | n.s. | 1.15 | n.s. | 0.38 | n.s. | 0.43 | n.s. | 0.60 | n.s. | 1.40 | n.s. |
| TF-RSA | 1.08 | n.s. | 0.03 | n.s. | 0.29 | n.s. | 0.01 | n.s. | 0.82 | n.s. | 0.38 | n.s. | 1.22 | n.s. |
| Secondary outcome measures | ||||||||||||||
| Heart rate | 0.07 | n.s. | 9.01 | .004 | 2.20 | n.s. | 0.07 | n.s. | 1.47 | n.s. | 1.10 | n.s. | 1.78 | n.s. |
| Tidal volume | 1.13 | n.s. | 0.03 | n.s. | 0.63 | n.s. | 0.12 | n.s. | 2.68 | n.s. | 0.01 | n.s. | 0.36 | n.s. |
| Tidal volume instability | 0.40 | n.s. | 0.28 | n.s. | 1.74 | n.s. | 2.27 | n.s. | 1.34 | n.s. | 0.58 | n.s. | 0.33 | n.s. |
| % RC | 0.26 | n.s. | 0.00 | n.s. | 1.90 | n.s. | 0.11 | n.s. | 1.56 | n.s. | 1.83 | n.s. | 1.07 | n.s. |
| qDEEL | 0.03 | n.s. | 0.03 | n.s. | 0.31 | n.s. | 3.36 | n.s. | 1.65 | n.s. | 0.73 | n.s. | 0.47 | n.s. |
| Respiratory rate | 0.60 | n.s. | 0.17 | n.s. | 2.80 | n.s. | 3.45 | n.s. | 0.70 | n.s. | 0.31 | n.s. | 0.55 | n.s. |
| Minute ventilation | 0.49 | n.s. | 0.24 | n.s. | 1.53 | n.s. | 1.82 | n.s. | 1.40 | n.s. | 0.43 | n.s. | 0.12 | n.s. |
| F/Vt | 2.15 | n.s. | 0.62 | n.s. | 0.90 | n.s. | 0.17 | n.s. | 2.01 | n.s. | 0.05 | n.s. | 0.47 | n.s. |
| Ti/Tt | 0.00 | n.s. | 7.95 | .006 | 4.08 | .001 | 2.27 | n.s. | 0.62 | n.s. | 1.62 | n.s. | 0.81 | n.s. |
Note. ACCM = accelerometer motion indicator; RR SD(mean) = SD of the mean of consecutive 10 sec long interbeat interval segments, RR SD(SD) = SD of the SD of consecutive 10 sec long interbeat interval segments; NA = negative affect; PA = positive affect; VLF = very low frequency; LF = low frequency; HF = high frequency; TF-RSA = transfer function respiratory sinus arrhythmia; HR = heart rate; TV = tidal volume; % RC = percent rib cage contribution to tidal volume ratio; qDEEL = breath by breath differences of end-expiratory lung volume; MV = minute ventilation; F/Vt = rapid shallow breathing index (respiratory rate / tidal volume); Ti/Tt = fractional inspiratory time.
To exclude bias in the spectral analysis, stationarity of the RR-interval time series was quantified by (a) the SD of the mean of consecutive 10 sec data segments and (b) the SD of SDs of these segments (Pincus, Gladstone, & Ehrenkranz, 1991) and tested for differences in gender, group, or time. For the SD of the SD of the segments, there were no main or interaction effects, but the mixed effects model analysis on the SD of the mean of these segments indicated a significant gender effect (see Table 2). 10% of data points had to be excluded for the TF-RSA analysis because spectral coherence was below 0.5. A mixed model analysis did not indicate differences in coherence between groups, gender, or time points (see Table 2).
Outcomes
Table 2 shows the mixed model results by group, time, gender, and their interactions for the major psychophysiological variables, cortisol, and affect scores. There were significant overall differences between groups in NA and PA. Depressed participants reported significantly greater NA and less PA than nondepressed (Figure 1, Figure 2). The group × gender effect for PA was due to an increase in PA in nondepressed females throughout the day. There was a time effect for cortisol, but cortisol did not differ between groups (Figure 3). An ANOVA of cortisol 30 min after waking with the factors group and gender did not indicate any significant effects [group (F(1, 61) = 0.48, n.s.), gender effect (F(1, 61) = 0.15, n.s.), or interaction (F(1, 61) = 0.37, n.s.)]. There was no group effect for any of the spectral measures. There was a time effect for VLF and LF indicating that these measures declined in the morning, reached a minimum in the early afternoon, and increased towards the evening. A gender effect was a result of males having higher VLF during the assessment.
Figure 1.
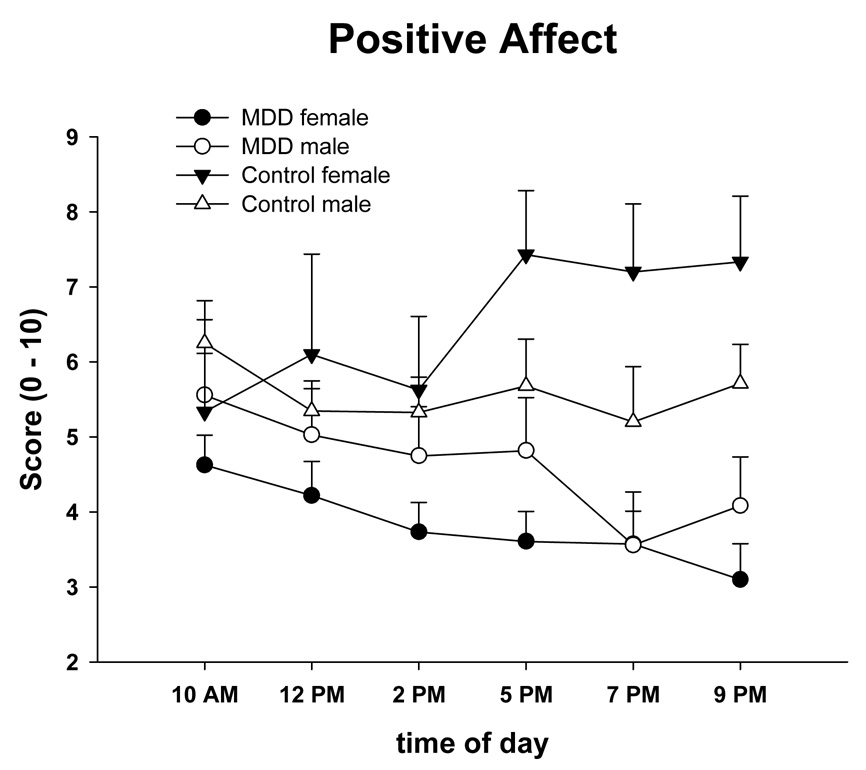
Means and standard errors of State Positive Affect (PANAS) across the day in male and female depressed and nondepressed subjects at risk for CVD.
Figure 2.
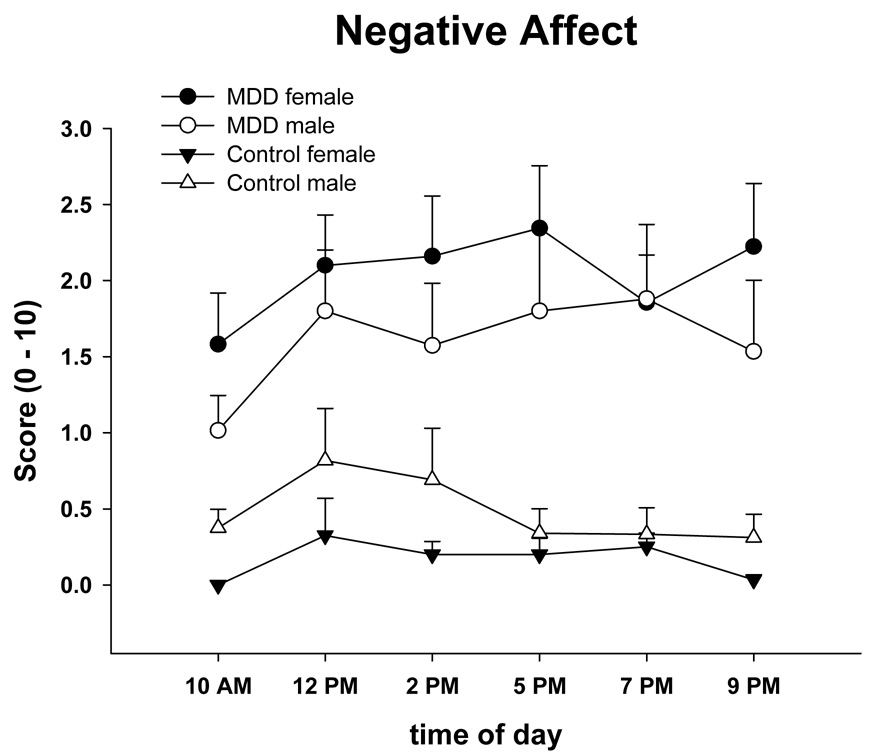
Means and standard errors of State Negative Affect (PANAS) across the day in male and female depressed and nondepressed subjects at risk for CVD.
Figure 3.
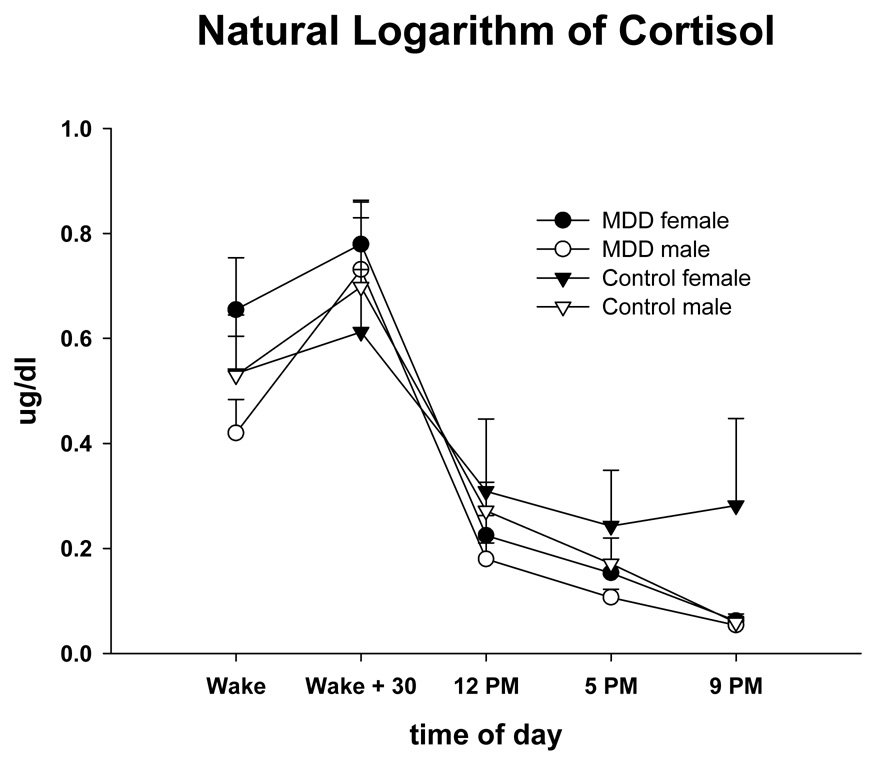
Means and standard errors of Log Cortisol across the day in male and female depressed and nondepressed subjects at risk for CVD.
Of the secondary psychophysiological outcome measures, only fractional inspiratory time showed group or time effects. Fractional inspiratory time increased throughout the day, and was larger in male than female participants. Females exhibited higher HR than males, which is an often replicated finding in the psychophysiological literature. There were no effects for tidal volume, tidal volume instability, respiratory rate, minute volume, percent rib cage contribution to tidal volume, breath-by-breath differences of end-expiratory lung volume, the rapid shallow breathing index (respiratory rate / tidal volume), or total breath time.
The results of the random coefficient models for the relationship between affect and HRV are depicted in Table 3. For TF-RSA and HF there were significant effects for centered NA. Higher levels of negative emotions were associated with lower HRV. There were significant or marginally significant terms of each spectral measure for centered NA × Group, with higher NA corresponding to lower HRV in nondepressed but not depressed individuals. PA was not associated with HRV.
Table 3.
Results of random coefficient models for diary entries and heart rate variability variables
| Affect | Group | Affect × Group | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Model ln VLF = cNA + group + group × cNA | 3.54 | (0.06) | 0.88 | n.s. | 7.36 | 0.007 |
| Model ln LF = cNA + group + group × cNA | 2.19 | n.s. | 0.00 | n.s. | 3.42 | (0.07) |
| Model ln HF = cNA + group + group × cNA | 6.68 | 0.01 | 1.48 | n.s. | 3.94 | 0.05 |
| Model TF-RSA = cNA + group + group × cNA | 5.69 | 0.02 | 3.00 | (0.09) | 3.09 | (0.08) |
| Model ln VLF = cPA + group + group × cPA | 1.18 | n.s. | 1.40 | n.s. | 2.71 | n.s. |
| Model ln LF = cPA + group + group × cPA | 0.43 | n.s. | 1.67 | n.s. | 0.40 | n.s. |
| Model ln HF = cPA + group + group × cPA | 0.60 | n.s. | 0.07 | n.s. | 2.56 | n.s. |
| Model TF-RSA = cPA + group + group × cPA | 0.56 | n.s. | 0.03 | n.s. | 1.56 | n.s. |
Note. cNA = centered negative affect; cPA = centered positive affect; VLF = very low frequency; LF = low frequency; HF = high frequency; TF-RSA = transfer function respiratory sinus arrhythmia. p values in parentheses indicate marginal significance (p < 0.1).
Discussion
The most important finding of this study is a lack of HRV, cortisol, or respiratory differences between depressed and nondepressed at-risk patients during daily life, despite the fact that the groups differed in positive and negative mood states in the expected directions. These findings are in contrast to our previously reported stress findings in the laboratory, which indicated HRV and cortisol differences (Taylor et al., 2006), and to other laboratory investigations (e.g., Agelink et al., 2002; Carney et al., 2005; Carney et al., 2001). Recent results from the Heart and Soul study (Gehi, Mangano, Pipkin, Browner, & Whooley, 2005) support our HRV findings: In a cross-sectional design with 873 CVD patients (195 depressed), the authors did not find a relationship between depression and time-or frequency-domain measures of HRV that were measured by 24-hour ambulatory electrocardiography. Importantly, our novel ambulatory study design excludes the possibility that measurement bias due to physical activity influenced the results. As would be expected, NA was higher and PA lower in depressed compared to non-depressed subjects, although neither followed the predicted circadian pattern of negative affect being worse in the evening.
The second finding of the study is that high levels of negative emotions are related to low HRV in nondepressed but not in depressed individuals. The findings in the nondepressed sample are consistent with Bacon et al. (2004) who found that high NA was associated with lower HRV in nondepressed patients at risk for CVD. If NA affects HRV, then depressed mood, which is associated with prolonged NA, should have an even greater effect on HRV and we had expected to see overall differences between the depressed and nondepressed subjects. One explanation for a lack of association of HRV and temporary affect in depressed patients may be that constant high NA disturbs the normal circadian connection between state affectivity and autonomic nervous system control, e.g. by a ceiling effect on regulatory processes. The reduced range of NA may also have limited our statistical power to determine differences. It is also interesting that negative but not positive affect was related to low RSA in nondepressed subjects. This finding is consistent with others that have found that positive and negative affect are relatively independent constructs, e.g. positive affect is not simply the absence of negative affect (Strachowski et al., 2007).
As often reported (e.g., Clow, Thorn, Evans, & Hucklebridge, 2004; Wust et al., 2000), cortisol peaked in the early morning following awakening and then gradually declined over the course of the day for both depressed and nondepressed subjects. There was no indication of hypercortisolism in depressed subjects in this sample. Maes and colleagues (1994) suggested that increased activation of the HPA-axis is more prominent in severe than moderate depression. Further, hypercortisolism in depression has been most consistently found in subjects with "severe forms of depression", for instance those with psychotic features (Keller et al., 2006), which none of our subjects had, or with melancholia (Gold & Chrousos, 2002; Stewart, Quitkin, McGrath, & Klein, 2005). Nonpsychotic subjects with more moderate levels of depression may not show hypercortisolemia (Keller et al., 2006). As indicated by the mean Hamilton scores, depression in our study was moderate. As previoulsly noted we did find a hypocortisol response to stress during psychological stress testing conducted in the afternoon (Taylor et al., 2006).
Our study is unique in several ways. It is the first comprehensive attempt to capture circadian changes of HRV, cortisol, and mood in depressed and nondepressed participants at risk for CVD. Although participants were monitored ambulatorily, physical activity – a potential confound when assessing HRV measures as indices of autonomic group differences – was controlled for during the physiological assessment. Since so little literature is available on circadian variation of mood and cardiopulmonary variables in this patient group, we did not restrict ourselves to hypothesis testing, but explored an array of seldomly studied respiratory measures. However, these did not differ between groups. There are, however, a number of limitations in our study. Our groups were not balanced for gender or race. Outside the laboratory, we had less control over whether participants complied with instructions and sat quietly during the 5-min assessments. However, segments for which sensors indicated significant movement or talking were excluded from the analyses. Some complex group × gender and gender × time interactions emerged which need to be considered in future research using a more gender balanced design. We could not take the participating high risk participants off their medications, many of which are known to affect the cardiovascular system. We excluded smokers from the study and thus our results may be limited to non-smokers. We did not include nondepressed individuals without CVD, and thus, could not compare our groups to healthy controls. Some stationarity assumptions were violated in the spectral analysis of traditional HRV; however, groups did not differ in this respect.
Statistical power needs to be considered whenever hypothesized differences fail to reach significance. This study was adequately powered to inform us whether HRV differed between depressed and non-depressed individuals. We report data from 46 depressed and 19 non-depressed patients, whereas Carney’s laboratory found differences between groups during 24-hour ambulatory electrocardiographic monitoring of 19 depressed and 19 nondepressed patients (Carney et al., 1995). We also had enough power to detect cortisol differences between groups: Gotthardt et al. (1995) found cortisol differences between groups in 20 depressed and 20 nondepressed participants. We are less certain about the correlational analyses. We did not perform a pilot study before this project (for cautionary notes regarding the use of pilot studies for power calculations, see Kraemer, Mintz, Noda, Tinklenberg, & Yesavage, 2006), and thus the analyses need to be regarded as exploratory.
In conclusion, depressed and nondepressed participants at risk for CVD differ in PA and NA but not in cortisol or cardiopulmonary variables measured at several times during the day. Furthermore, the same sample demonstrated an impaired cortisol response to psychological stress, and a lower TF-RSA, although their cardiovascular reactivity was otherwise similar to controls (Taylor et al., 2006). HRV was related to NA in controls but not among depressed patients with CVD risk. In light of previous findings, the data suggest that different populations of depression may have different HPA and autonomic response patterns and that these responses also vary by time of day and situation (e.g. laboratory stress testing). Studies with larger samples, well characterized by length and treatment of depression and comorbid disorders (such as history of post-traumatic stress disorder) are needed to clarify these matters.
Acknowledgement
We would like to thank Helena C. Kraemer and Eric Neri for consultation regarding the statistical analyses. In addition, we would like to thank research assistants Allyson DeLorenzo and Christine Celio, and the volunteers participating in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agelink MW, Boz C, Ullrich H, Andrich J. Relationship between major depression and heart rate variability. Clinical consequences and implications for antidepressive treatment. Psychiatry Research. 2002;113:139–149. doi: 10.1016/s0165-1781(02)00225-1. [DOI] [PubMed] [Google Scholar]
- Bacon SL, Watkins LL, Babyak M, Sherwood A, Hayano J, Hinderliter AL, Waugh R, Blumenthal JA. Effects of daily stress on autonomic cardiac control in patients with coronary artery disease. American Journal of Cardiology. 2004;93:1292–1294. doi: 10.1016/j.amjcard.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Bagiella E, Sloan RP, Heitjan DF. Mixed-effects models in psychophysiology. Psychophysiology. 2000;37:13–20. [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;41:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005:1–4. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Brown E, Varghese F, McEwen B. Association of depression with medical illness: does cortisol play a role? Biological Psychiatry. 2004;55:1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Freedland KE, Stein PK, Howells WB, Berkman LF, Watkins LL, Czajkowski SM, Hayano J, Domitrovich PP, Jaffe AS. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Archives of Internal Medicine. 2005;165:1486–1491. doi: 10.1001/archinte.165.13.1486. [DOI] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O'Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Sheps DS. Depression is a risk factor for mortality in coronary heart disease. Psychosomatic Medicine. 2004;66:799–801. doi: 10.1097/01.psy.0000146795.38162.b1. [DOI] [PubMed] [Google Scholar]
- Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. American Journal of Cardiology. 1995;76:562–564. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- Clark L, Watson D, Leeka J. Diurnal Variation in the Positive Affects. Motivation and Emotion. 1989;13:205–234. [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. International Journal of Psychophysiology. 2000;37:121–133. doi: 10.1016/s0167-8760(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Freedland KE, Skala JA, Carney RM, Raczynski JM, Taylor CB, Mendes de Leon CF, Ironson G, Youngblood ME, Krishnan KR, Veith RC. The Depression Interview and Structured Hamilton (DISH): rationale, development, characteristics, and clinical validity. Psychosomatic Medicine. 2002;64:897–905. doi: 10.1097/01.psy.0000028826.64279.29. [DOI] [PubMed] [Google Scholar]
- Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Archives of General Psychiatry. 2005;62:661–666. doi: 10.1001/archpsyc.62.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese-Davis J, Wilhelm FH, Conrad A, Abercrombie HC, Sephton S, Yutsis M, Neri E, Taylor CB, Kraemer HC, Spiegel D. Depression and stress reactivity in metastatic breast cancer. Psychosomatic Medicine. 2006;68:675–683. doi: 10.1097/01.psy.0000238216.88515.e5. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Molecular Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gotthardt U, Schweiger U, Fahrenberg J, Lauer CJ, Holsboer F, Heuser I. Cortisol, ACTH, and cardiovascular response to a cognitive challenge paradigm in aging and depression. American Journal of Physiology. 1995;268:R865–R873. doi: 10.1152/ajpregu.1995.268.4.R865. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Grossman P, Wilhelm FH, Spoerle M. Respiratory sinus arrhythmia, cardiac vagal control, and daily activity. American Journal of Physiology. 2004;287:H728–H734. doi: 10.1152/ajpheart.00825.2003. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Flores B, Gomez RG, Solvason HB, Kenna H, Williams GH, Schatzberg AF. Cortisol circadian rhythm alterations in psychotic major depression. Biological Psychiatry. 2006;60:275–281. doi: 10.1016/j.biopsych.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Blasey CM. Centering in regression analyses: A strategy to prevent errors in statistical inference. International Journal of Methods in Psychiatric Research. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Archives of General Psychiatry. 2006;63:484–489. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- Maes M, Calabrese J, Meltzer HY. The relevance of the in- versus outpatient status for studies on HPA-axis in depression: spontaneous hypercortisolism is a feature of major depressed inpatients and not of major depression per se. Progress in Neuropsychopharmacology and Biological Psychiatry. 1994;18:503–517. doi: 10.1016/0278-5846(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Murray G, Allen N, Trinder J. Mood and the circadian system: investigation of a circadian component in positive affect. Chronobiology International. 2002;19:1151–1169. doi: 10.1081/cbi-120015956. [DOI] [PubMed] [Google Scholar]
- Musselman D, Evans D, Nemeroff C. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Archives of General Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Gladstone IM, Ehrenkranz RA. A regularity statistic for medical data analysis. Journal of Clinical Monitoring. 1991;7:335–345. doi: 10.1007/BF01619355. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatric Clinics of North America. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Respiratory sinus arrhythmia as a predictor of outcome in major depressive disorder. Journal of Affective Disorders. 2002;71:265–272. doi: 10.1016/s0165-0327(01)00406-2. [DOI] [PubMed] [Google Scholar]
- Rusting C, Larsen R. Diurnal patterns of unpleasant mood: associations with neuroticism, depression, and anxiety. Journal of Personality. 1998;66:85–103. doi: 10.1111/1467-6494.00004. [DOI] [PubMed] [Google Scholar]
- Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: Unique insights into cardiovascular regulation. American Journal of Physiology. 1991;261:H1231–H1245. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- Schwarz AM, Schachinger H, Adler RH, Goetz SM. Hopelessness is associated with decreased heart rate variability during championship chess games. Psychosomatic Medicine. 2003;65:658–661. doi: 10.1097/01.psy.0000075975.90979.2a. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Boni SM, Paik M, Bigger JT, Jr., Steinman RC, Gorman JM. Effect of mental stress throughout the day on cardiac autonomic control. Biological Psychology. 1994;37:89–99. doi: 10.1016/0301-0511(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JW, Quitkin FM, McGrath PJ, Klein DF. Defining the boundaries of atypical depression: evidence from the HPA axis supports course of illness distinctions. Journal of Affective Disorders. 2005;86:161–167. doi: 10.1016/j.jad.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Strachowski D, Khaylis A, Conrad A, Neri E, Spiegel D, Taylor CB. The effects of cognitive behavior therapy on depression in older patients with cardiovascular risk. Depression and Anxiety. 2007 doi: 10.1002/da.20302. [DOI] [PubMed] [Google Scholar]
- Strike PC, Wardle J, Steptoe A. Mild acute inflammatory stimulation induces transient negative mood. Journal of Psychosomatic Research. 2004;57:189–194. doi: 10.1016/S0022-3999(03)00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg NO, Dahlback GO, Gustafsson PM. Evaluation of various models for respiratory inductance plethysmography calibration. Journal of Applied Physiology. 1993;74:1206–1211. doi: 10.1152/jappl.1993.74.3.1206. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Conrad A, Wilhelm FH, Neri E, DeLorenzo A, Kramer MA, Giese-Davis J, Roth WT, Oka R, Cooke JP, Kraemer H, Spiegel D. Psychophysiological and cortisol responses to psychological stress in depressed and nondepressed older men and women with elevated cardiovascular disease risk. Psychosomatic Medicine. 2006;68:538–546. doi: 10.1097/01.psy.0000222372.16274.92. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76:820–838. [Google Scholar]
- Wilhelm F, Roth W, Sackner M. The LifeShirt: An advanced system for ambulartory measurement of respiratory and cardiac function. Behavior Modification. 2003;27:671–691. doi: 10.1177/0145445503256321. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko N, Schommer N, Kirschbaum C. The cortisol awakening response-normal values and confounds. Noise Health. 2000;2:79–88. [PubMed] [Google Scholar]


