Abstract
This study examines the association of keratotic skin lesions with the development of skin cancer in 915 solid organ-transplant recipients in five European countries. In a hospital-based case–control study, cases with squamous- and basal-cell carcinoma were compared with controls without skin cancer. Questionnaires, scrutiny of medical charts, and skin examination were delivered according to a standardized protocol. Keratotic skin lesions and viral warts were counted on different body sites. Keratotic skin lesions were strongly associated with an increased risk of squamous-cell carcinoma, with adjusted odds ratios of 4.1 (2.4;7.0) and 12.1 (6.1;24) for 1–49 and 50 and more keratotic skin lesions compared with no lesions, respectively. Keratotic skin lesions were also associated with basal-cell carcinoma with adjusted odds ratios of 2.9 (1.7;4.9) and 4.0 (1.7;9.2) for 1–49 and 50 and more lesions, respectively. Lighter skin types and painful sunburns were also significantly associated with an increased risk of squamous- and basal-cell carcinoma. Keratotic skin lesions are strongly associated with skin cancer and are, thus, an important clinical criterion for identifying those organ-transplant recipients at an increased risk of skin cancers who should be offered more intensive skin surveillance.
INTRODUCTION
Persistent viral warts, premalignant actinic keratoses, and skin cancers are common cutaneous lesions in organ-transplant recipients (Boyle et al., 1984; Bouwes Bavinck et al., 1991; Berg and Otley, 2002; Euvrard et al., 2003).
The risk of squamous- and basal-cell carcinomas in organ-transplant recipients is markedly increased compared with the normal population (Hartevelt et al., 1990; Bouwes Bavinck et al., 1996; Jensen et al., 2000; Lindelof et al., 2000; Naldi et al., 2000; Adami et al., 2003; Moloney et al., 2006). Standardized incidence ratios are increased up to 250 for squamous-cell carcinoma (Hartevelt et al., 1990; Jensen et al., 2000; Lindelof et al., 2000; Adami et al., 2003; Moloney et al., 2006) and are around 10 for basal-cell carcinoma (Hartevelt et al., 1990). Because of an excess of squamous-cell carcinomas, the ratio of squamous- to basal-cell carcinoma in these individuals is reversed as compared with that in the general population (Ramsay et al., 2002; Euvrard et al., 2003; Fortina et al., 2004).
Sex, age, skin type, and time after transplantation are important intrinsic risk factors for actinic keratoses and skin cancer in organ-transplant recipients. Sun exposure has been recognized as the most important environmental risk factor for both types of lesions (Boyle et al., 1984; Bouwes Bavinck et al., 1991, 1993). Smoking (Ramsay et al., 2000; De Hertog et al., 2001; Freedman et al., 2003; Rosenquist et al., 2005; Vallejo et al., 2005) and alcohol consumption (Freedman et al., 2003; Lindelof et al., 2003; Rosenquist et al., 2005) have also been implicated as possible risk factors for keratinocytic skin cancer.
The number of keratotic skin lesions is a strong indicator of the risk of skin cancer in individual recipients (Boyle et al., 1984; Shuttleworth et al., 1987; Blohme and Larko, 1990; Bouwes Bavinck et al., 1993; de Jong-Tieben et al., 2000). In a small Dutch study from 1993 with 36 patients with and 101 without skin cancer, the risk of skin cancer was increased approximately 5-fold among those with 50–99 lesions and 20-fold among people with more than 100 lesions compared with patients who had less than 50 actinic keratoses (Bouwes Bavinck et al., 1993). The purpose of this study was to validate this observation in a much larger patient cohort, across multiple countries, and in the context of far more rigorous evaluation of other potentially interacting risk factors. Furthermore, the risk of keratotic skin lesions on the development of skin cancer has not been systematically studied in other countries and Southern countries have never been compared with Northern countries.
Beta-papillomaviruses, formerly called epidermodysplasia verruciformis (EV)-associated human papillomavirus types, have been frequently detected in actinic keratoses and other keratotic skin lesions of organ-transplant recipients (de Jong-Tieben et al., 1995, 2000; Berkhout et al., 2000; Harwood et al., 2000). Numerous studies have suggested a possible causal role of beta-papillomavirus infections in the pathogenesis of skin cancer, either directly, or in conjunction with sun exposure (Berkhout et al., 2000; de Jong-Tieben et al., 2000; Harwood et al., 2000; Jackson et al., 2000; Iftner et al., 2002; Feltkamp et al., 2003; Masini et al., 2003; Struijk et al., 2003; Bouwes Bavinck and Feltkamp, 2004; Karagas et al., 2006).
In this study, we investigated associations between a variety of risk factors (including sun exposure, other lifestyle factors, keratotic skin lesions, and viral warts) with the development of squamous- and basal-cell carcinoma in a large group of organ-transplant recipients from five European countries. We also examined associations between sun exposure and lifestyle factors and the development of keratotic skin lesions and viral warts.
RESULTS
Baseline characteristics of the study population
The characteristics of the study populations according to the presence of skin cancer are presented in Tables 1 and S1.
Table 1. Baseline characteristics of the population.
| All countries together1 |
No skin cancer (N=560) |
Squamous-cell carcinoma (with or without BCC) (N=224) |
Basal-cell carcinoma (with no SCC) (N=131) |
||
|---|---|---|---|---|---|
| Number male | |||||
| N (%) | 384 (68.6) | 171 (76.3) | P=0.031 | 96 (73.3) | P=0.292 |
| Age (years) | |||||
| Mean (SD) | 52.4 (12.0) | 59.8 (9.2) | P<0.001 | 56.5 (10.6) | P<0.001 |
| Min–max | 15.7–77.9 | 34.1–80.9 | 26.9–77.6 | ||
| Time since Tx (years) | |||||
| Mean (SD) | 11.9 (7.3) | 15.8 (7.6) | P<0.001 | 12.3 (7.0) | P=0.559 |
| Min–max | 0.2–35.7 | 1.8–36.8 | 1.3–37.0 | ||
BCC, basal-cell carcinoma; SCC, squamous-cell carcinoma; SD, standard deviation; Tx, transplantation.
The data for the five countries are provided separately in >Table S1.
A total of 224 patients (24%) had at least one squamous-cell carcinoma, with the number varying between 1 and 45 (mean, 3.5; median, 1). Of these patients, 93 also had between 1 and 27 basal-cell carcinomas (mean, 3.2; median, 2). A total of 131 patients (14%) had basal-cell carcinoma only, with the number of lesions varying between 1 and 18 (mean, 1.7; median, 1).
The percentages of males without skin cancer varied between 63% in the United Kingdom and 80% in Italy, in line with the higher percentage of males with renal dysfunction in the general population. Despite our attempts to match for sex, the proportion of males was significantly higher among patients with squamous-cell carcinoma, when all centers were considered together (Table 1). The cases were also significantly older and the time period after transplantation in these patients was significantly longer in all countries with the exception of France (Tables 1 and S1).
A total of 789 (86%) patients had received a renal transplant although this varied somewhat by country. In Germany and the United Kingdom, above 97% of participants had received a renal transplant, whereas in The Netherlands 33 (18%) had received a combined kidney and pancreas transplant. Heart transplants were more frequent in France and Italy with 42 (32%) and 34 (18%) receiving heart transplants in these countries, respectively. The different organ transplants were equally distributed among the recipients with and without skin cancer. The number of renal transplants ranged between 1 and 4 transplants, which were also equally distributed among the recipients with and without skin cancer. Separate analyses of the different organs transplanted did not result in significantly different outcomes.
Immunosuppressive regimens
The immunosuppressive regimens differed considerably according to country and to the type of organ transplanted. In The Netherlands, the most commonly used immunosuppressive regimen was prednisone and azathioprine (38% of participants) followed by prednisone and cyclosporine (24%). In the United Kingdom, France, and Italy, a preference for triple therapy using prednisone/prednisolone, azathioprine, and cyclosporine was seen, with this combination being used in above 30% of participants.
The 42 heart-transplant recipients in France were most commonly immunosuppressed with prednisolone and cyclosporine (31%), whereas in Italy triple therapy was more frequent, with prednisone/prednisolone, azathioprine, and cyclosporine accounting for 24%, and prednisone, cyclosporine, and mycofenolatemofetil a further 27%. The 39 kidney–pancreas transplant recipients in The Netherlands and France were mainly immunosuppressed with triple therapy, either prednisone/prednisolone, azathioprine, and cyclosporine (30% in The Netherlands, 17% in France) or prednisone/prednisolone, cyclosporine, and mycofenolatemofetil (42% in The Netherlands, 67% in France).
The time from first transplantation was the most important determinant for the immunosuppression given. In particular, azathioprine in any combination was far more common in patients who were transplanted before 1985. Patients who had used azathioprine in any combination had no significantly increased risk of squamous-cell carcinoma compared with those who had not used this drug with an adjusted OR of 1.2 (0.84;1.8). The adjusted OR for an increased risk of basal-cell carcinoma was 1.2 (0.78;1.9).
Risk factors for verrucae vulgares and plantar warts
The type of organ transplant was not associated with the development of verrucae vulgares and plantar warts. Although the non-adjusted OR was 2.4 (1.6;3.6) for patients receiving a renal transplant compared with another transplant (kidney–pancreas, heart), the association was completely lost after adjustment with an adjusted OR of 1.0 (0.62;1.6). Skin phototype and education were not associated with the development of verrucae vulgares and plantar warts with adjusted ORs of 1.0 (0.72;1.4) when medium skin was compared with olive skin, 0.66 (0.43;1.0) when fair skin was compared with olive skin, and 1.1 (0.82;1.25) when high education was compared with low and middle education, respectively. Before adjustment there was no evidence of an association.
There was some suggestion for a negative association between painful sunburns and having one or more verrucae vulgares and/or plantar warts. The non-adjusted ORs for 1–4 and 5 and more sunburns, respectively, compared with no sunburns were 0.77 (0.58;1.0) and 0.61 (0.40;0.92) and the adjusted ORs 0.81 (0.58;1.1) and 0.74 (0.46;1.2). There was no association between chronic and weekend sun exposure and the presence of verrucae vulgares and/or palmoplantar warts.
There was no association between smoking and the presence of verrucae vulgares and/or plantar warts with adjusted ORs 0.88 (0.63;1.2) and 1.3 (0.77;2.0), respectively. Similarly, there was no association between consumption of alcohol and the presence of verrucae vulgares and/or plantar warts with adjusted ORs of 0.94 (0.66;1.3) for low alcohol consumers and 0.64 (0.37;1.1) for those who consumed high amounts of alcohol, compared with those who never drank alcohol.
Risk factors for keratotic skin lesions
The type of organ transplant was not associated with the development of keratotic skin lesions. A lighter skin type, however, was significantly associated with the presence of one or more keratotic lesions with adjusted ORs, using olive-skinned people as the reference group, of 1.4 (0.91;2.1) for medium skin and 2.8 (1.6;4.8) for fair skin. Although a higher level of education appeared to be associated with the development of keratotic skin lesions (OR 1.9, 95% confidence interval 1.5;2.6), this association disappeared after adjustment (OR 1.2, 95% confidence interval 0.82;1.8).
We observed a weak association between painful sunburns and the presence of one or more keratotic skin lesions with adjusted ORs of 1.4 (0.97;2.2) and 1.5 (0.84;2.6) for 1–4 and 5 and more sunburns, respectively, compared with no sunburns. Similarly, the association between high levels of chronic sun exposure and the presence of one or more keratotic skin lesions (OR 1.6, 95% confidence interval 1.2;2.1) was abrogated by adjustment (OR 0.87, 95% confidence interval 0.58;1.3). There was no association between weekend sun exposure and the presence of keratotic skin lesions with an adjusted OR of 0.91 (0.62;1.3).
Compared with never smokers, current smokers and ex-smokers were at an increased risk of developing keratotic skin lesions with ORs of 2.0 (1.2;3.1) and 1.8 (1.3;2.4), respectively. After adjustment these ORs also went down to 1.4 (0.75;2.6) for current smokers and 0.78 (0.53;1.2) for ex-smokers, with study center being primarily responsible for the reduction in the ORs. Within each study center there were no associations between smoking and developing keratotic skin lesions.
We observed an association between alcohol consumption and the presence of keratotic skin lesions with non-adjusted ORs using no alcohol consumption as the reference category of 2.8 (2.1;3.8) for recipients with low alcohol consumption and 3.4 (2.1;5.3) for those who consumed high levels of alcohol. However, after adjustment the odds decreased to 1.2 (0.75;1.8) for recipients with low alcohol consumption and 1.3 (0.70;2.4) for recipients with high alcohol consumption, respectively. Again, study center analysis caused this association to disappear. Analyses of the study centers separately did not result in consistent associations. There was a trend toward a positive association between alcohol consumption and the presence of keratotic skin lesions in Italy, no trend in The Netherlands, Germany, and France, and a trend toward a negative association in the UK.
Common and palmoplantar warts are not consistently associated with skin cancer
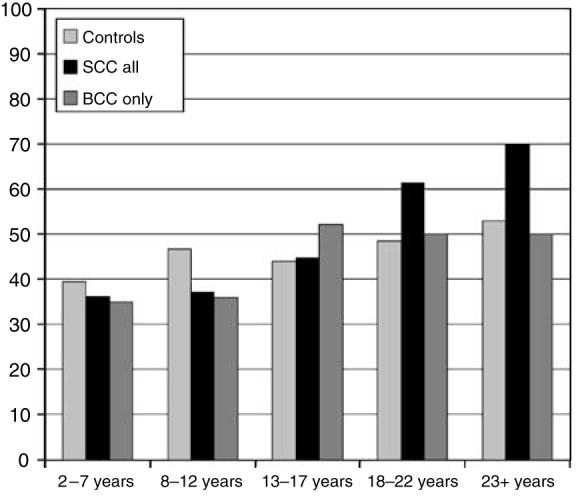
The distributions of verrucae vulgares and plantar warts according to the localization on the body are presented in Figure 1. A total of 282 out of 648 men (44%) and 127 out of 262 women (49%) had common and/or palmoplantar warts. The 409 recipients with verrucae vulgares were significantly younger (P=0.01) with a mean age of 54 (SD 12) years compared with the 501 recipients without verrucae vulgares who had a mean age of 56 (SD 11) years. The percentage of organ-transplant recipients with verrucae vulgares increased with increasing years after the transplantation (Figure 2). This was most marked in the patients with squamous-cell carcinoma, with almost a doubling in the proportion of people with verrucae vulgares (70% at 23+ years compared with 36% in the 2–7 years post-transplantation). The proportion increased from 39 to 53% in the recipients without skin cancer, and in those with basal-cell carcinoma increased from 35 to 50%, an increase of about 40% in both cases (Figure 2).
Figure 1. Distribution of skin lesions.
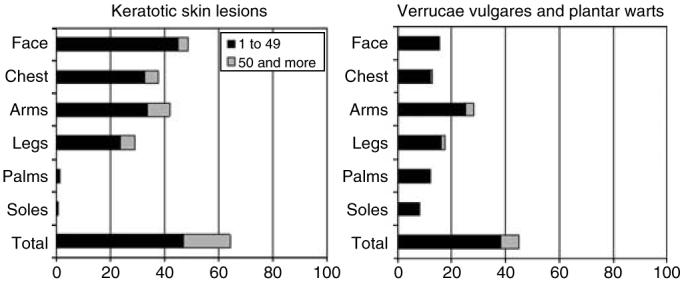
Percentages of patients with 1–49 and 50 and more keratotic skin lesions (consisting of actinic keratoses, seborrheic warts, plane warts, and hyperkeratotic papillomas), and verrucae vulgares or plantar (and palmar) warts according to localization on the body.
Figure 2. Percentages of patients with verrucae vulgares and plantar warts according to years after transplantation.
There appeared to be an association between the presence of verrucae vulgares and/or plantar warts and squamous-cell carcinoma but this association was only evident in The Netherlands and United Kingdom (Tables 2 and S2). Restricting the analysis to verrucae vulgares and plantar warts localized on the palms and soles; however, the association did not disappear completely, with an adjusted OR of 1.4 (0.87;2.2).
Table 2. Association between verrucae vulgares and plantar warts and skin cancer.
| No skin cancer |
Squamous-cell carcinoma (with or without BCC) |
Basal-cell carcinoma (with no SCC) |
|||||
|---|---|---|---|---|---|---|---|
|
All countries together1 |
N=557 |
N=222 |
Non-adjusted OR 1+ vs 0 (95% CI) |
Adjusted OR2 1+ vs 0 (95% CI) |
N=131 |
Non-adjusted OR 1+ vs 0 (95% CI) |
Adjusted OR2 1+ vs 0 (95% CI) |
| Verrucae vulgares and/or plantar ;warts | N (%) | N (%) | N (%) | ||||
| 0 | 311 (55.8) | 113 (50.9) | 77 (58.8) | ||||
| 1–49 | 225 (40.4) | 78 (35.1) | 1.2 (0.89;1.7) | 1.6 (1.1;2.3) | 46 (35.1) | 0.89 (0.60;1.3) | 1.0 (0.66;1.6) |
| 50 and more | 21 (3.8) | 31 (14.0) | 8 (6.1) | ||||
BCC, basal-cell carcinoma; CI, confidence interval; OR, odds ratio; SCC, squamous-cell carcinoma.
The data for the five countries are provided separately in Table S2.
Adjusted for age, sex, years after transplantation and study center.
We did not observe a consistent association between the presence of verrucae vulgares and basal-cell carcinoma (Tables 2 and S2 and Figure 2). There was also no association when performing analyses restricted to verrucae vulgares and plantar warts localized on the palms and soles.
Keratotic skin lesions are strongly associated with skin cancer
The distribution of keratotic skin lesions according to the localization on the body is also presented in Figure 1. More than 60% of all organ-transplant recipients had keratotic skin lesions that were present on all body sites with the exception of the palms and soles.
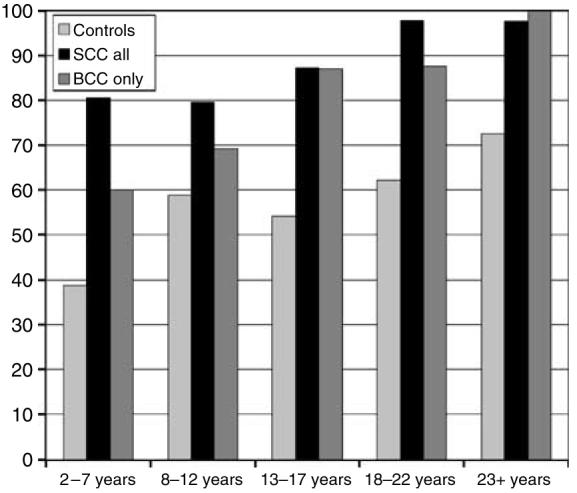
A total of 433 out of 648 (67%) men had keratotic skin lesions, compared with 150 out of 262 (57%) women. The 583 recipients with keratotic skin lesions were significantly older (P<0.0001) with a mean age of 57 (SD 10) years compared with the 327 recipients without keratotic skin lesions who had a mean age of 50 (SD 12) years. Among controls, the percentage with keratotic skin lesions increased with increasing years after the transplantation from 39% in the patients who were transplanted 2–7 years ago compared with 73% in the patients who were transplanted at least 23 years ago. These percentages increased from 81 to 98% and from 60 to 100% in the recipients with squamous- and basal-cell carcinoma, respectively (Figure 3).
Figure 3. Percentages of patients with keratotic skin lesions according to years after transplantation.
The organ-transplant recipients in The Netherlands and United Kingdom were more severely affected by keratotic skin lesions compared with the recipients in Germany, France, and Italy (Table S3). Nevertheless, there was a strong association between the presence of keratotic skin lesions and squamous-cell carcinoma in all five centers with adjusted ORs for all countries combined of 4.1 and 12.1 for 1–49 and 50 and more keratotic skin lesions compared with no keratotic skin lesions, respectively (Tables 3 and S3). There also appeared to be a statistically significant, but weaker association between the presence of keratotic skin lesions and basal-cell carcinoma, with adjusted ORs for all countries combined of 2.9 and 4.0 for 1–49 and 50 and more keratotic skin lesions compared with no keratotic skin lesions, respectively (Tables 3 and S3).
Table 3. Keratotic skin lesions are associated with skin cancer.
| No skin cancer | Squamous-cell carcinoma (with or without BCC) | Basal-cell carcinoma (with no SCC) | |||||
|---|---|---|---|---|---|---|---|
| Non-adjusted OR | Adjusted OR2 | Non-adjusted OR | Adjusted OR2 | ||||
| 1–49 vs 0 (95% CI) | 1–49 vs 0 (95% CI) | 1–49 vs 0 (95% CI) | 1–49 vs 0 (95% CI) | ||||
| All countries together1 | N=557 | N=222 | 50 + vs 0 (95% CI) | 50 + vs 0 (95% CI) | N=131 | 50 + vs 0 (95% CI) | 50 + vs 0 (95% CI) |
| Keratotic skin lesions3 | N (%) | N (%) | N (%) | ||||
| 0 | 267 (47.9) | 26 (11.7) | 34 (26.0) | ||||
| 1–49 | 231 (41.5) | 115 (51.8) | 5.1 (3.2;8.1) | 4.1 (2.4;7.0) | 79 (60.3) | 2.7 (1.7;4.2) | 2.9 (1.7;4.9) |
| 50–99 | 25 (4.5) | 25 (11.3) | 14.1 (8.3;24) | 12.1 (6.1;24) | 5 (3.8) | 1.4 (1.3;4.5) | 4.0 (1.7;9.2) |
| 100 and more | 34 (6.1) | 56 (25.2) | 13 (9.9) | ||||
BCC, basal-cell carcinoma; SCC, squamous-cell carcinoma.
The data for the five countries are provided separately in Table S3.
Adjusted for age, sex, years after transplantation and study center.
Keratotic skin lesions consist of actinic keratoses, seborrheic warts, and hyperkeratotic papillomas.
Additional risk factors for squamous-cell carcinoma
The associations of transplant type, skin phototype, education, sun exposure, smoking, and alcohol consumption with squamous-cell carcinoma are presented in Tables 4 and S4.
Table 4. Association of several risk factors with SCC (with or without BCC) and BCC (with no SCC).
| Squamous-cell carcinoma (with or without BCC) adjusted OR (95% CI)1 |
Basal-cell carcinoma (with no SCC) Adjusted OR (95% CI)1 |
|
|---|---|---|
| All countries together 2 | ||
| Transplant type | ||
| Only renal | 1.0 | 1.0 |
| Other types | 0.9 (0.52;1.5) | 0.9 (0.48;1.5) |
| Skin phototype | ||
| Olive | 1.0 | 1.0 |
| Medium | 2.0 (1.3;3.0) | 1.8 (1.1;2.8) |
| Fair | 1.8 (1.1;3.1) | 1.6 (0.90;2.9) |
| Education | ||
| Low and middle | 1.0 | 1.0 |
| High | 1.2 (0.80;1.7) | 1.8 (1.2;2.8) |
| Sunburn before 20 years | ||
| 0 | 1.0 | 1.0 |
| 1–4 | 1.7 (1.1;2.4) | 1.4 (0.87;2.2) |
| 5+ | 1.7 (0.96;2.9) | 2.0 (1.1;3.6) |
| Chronic sun exposure | ||
| 0–3 h | 1.0 | 1.0 |
| 4 and more | 0.92 (0.63;1.4) | 0.8 (0.51;1.2) |
| Weekend sun exposure | ||
| 0–3 h | 1.0 | 1.0 |
| 4 and more | 0.82 (0.57;1.2) | 1.1 (0.74;1.7) |
| Smoking | ||
| Never | 1.0 | 1.0 |
| Current | 1.0 (0.56;1.8) | 0.8 (0.42;1.6) |
| Ex | 0.6 (0.43;0.95) | 0.7 (0.45;1.1) |
| Alcohol | ||
| None | 1.0 | 1.0 |
| Low | 1.6 (0.92;2.7) | 0.8 (0.43;1.4) |
| High | 1.2 (0.72;1.9) | 0.7 (0.39;1.1) |
BCC, basal-cell carcinoma; SCC, squamous-cell carcinoma.
Adjusted for age, sex, years after transplantation and study center.
The complete data for the five countries are provided separately in Tables S4 and S5.
The type of organ transplant was not statistically and significantly associated with squamous-cell carcinoma. As might be expected, the distribution of skin phototype was significantly different among the five countries and the association with squamous-cell carcinoma varied according to country (Table S4). In the UK and Italy, recipients with squamous-cell carcinoma were significantly more likely to be fair-skinned than controls, but a significant association was not seen in other countries. When all centers were combined, there was approximately a 2-fold increased risk in those who had a medium or fair skin type compared with those with an olive skin type (Table 4). The level of education was not consistently associated with squamous-cell carcinoma in our study (Tables 4 and S4).
German transplant recipients had experienced fewer painful sunburns compared with the transplant recipients in other countries. With the exception of Germany, painful sunburns appeared to be associated with a slightly increased risk of squamous-cell carcinoma (Tables 4 and S4). To exclude the possibility that the association between painful sunburns and squamous-cell carcinoma was being confounded by an association between painful sunburns and basal-cell carcinoma, we also performed analyses restricted to persons with squamous-cell carcinoma without basal-cell carcinoma. The adjusted ORs were 1.6 (1.0;2.6) and 1.5 (0.79;2.9) for 1–4 painful sunburns and 5 and more painful sunburns compared with no painful sunburns, respectively, for all centers together. Restricting the analyses to persons with both squamous- and basal-cell carcinoma resulted in adjusted ORs of 1.9 (1.1;3.2) and 1.8 (0.86;4.0), respectively. In Italy, there was a trend toward increasing risk of squamous-cell carcinoma with high chronic and weekend sun exposure (Table S4), but this was not seen in other centers and was independent of gender.
The highest percentages of current and ex-smokers were in France (68.5%) and The Netherlands (64.0%), the lowest in Italy (48.5%) and Germany (28.6%). There was a trend for a positive association between smoking and squamous-cell carcinoma in The Netherlands and Germany, but a trend for a negative association in the United Kingdom, France, and Italy. Combining the data of the five countries did not show an increased risk for either type of skin cancer among smokers or ex-smokers (Tables 4 and S4) and there was no difference between men and women.
Alcohol consumption also varied substantially between the countries, ranging from 91% consumption in the controls in France to 22% alcohol consumption in the controls in Germany. A positive association between alcohol consumption and squamous-cell carcinoma was observed in The Netherlands, the United Kingdom, and Germany, as opposed to a negative association in France and no consistent association in Italy (Table S4). In France some patients were reluctant to admit alcohol consumption and they declined to supply data about the number of glasses of alcohol consumption per week, which may have influenced the data acquisition on alcohol consumption in France. Analyses using the number of alcohol years instead of the subclassification of no, low, and high alcohol consumption resulted in a similar outcome. Stratification by gender did not significantly alter the findings.
Additional risk factors for basal-cell carcinoma
The associations of transplant type, skin phototype, education, sun exposure, smoking, and alcohol consumption with basal-cell carcinoma are presented in Tables 4 and S5.
The type of organ transplant was not significantly associated with basal-cell carcinoma. There was a trend such that patients with basal-call carcinoma were more often fair skinned than the control patients, but the difference was less pronounced compared with the patients with squamous-cell carcinoma (Tables 4, S4 and S5). A higher level of education was significantly positively associated with the development of basal-cell carcinoma (Tables 4 and S5).
With the exception of Germany and France, painful sunburns appeared to be associated with a slightly increased risk for basal-cell carcinoma (Table S5), but chronic and weekend sun exposure did not (Table S5).
A positive association between alcohol consumption and basal-cell carcinoma was only observed in The Netherlands. Negative associations were found in France and Italy and inconsistent associations in the United Kingdom and Germany.
DISCUSSION
This case–control study of 915 organ-transplant recipients spanning five European countries represents the largest yet published to address clinical risk factors associated with post-transplant skin carcinogenesis. We have confirmed previous observations of a high burden of keratotic skin lesions, viral warts, and skin cancer in this patient population (Boyle et al., 1984; Hartevelt et al., 1990; Bouwes Bavinck et al., 1993; Naldi et al., 2000; Berg and Otley, 2002; Euvrard et al., 2003; Moloney et al., 2006). In addition, we have identified keratotic skin lesions as being strongly associated with skin cancer risk, the other major independent risk factors being childhood sunburn and skin phototype.
Risk factors for keratotic skin lesions, verrucae vulgares, and plantar warts
Increasing age, increasing time after the transplantation, male sex, and lighter skin type were the most important risk factors for the presence of keratotic skin lesions. There were considerable differences between the five countries regarding the presence and number of keratotic skin lesions, which mainly reflected differences in time period after transplantation and skin phototype. Painful sunburns and chronic and recreational sun exposure appeared to be risk factors for the development of keratotic skin lesions, but were not independent of sex, older age, and longer time period since transplantation of the patients with keratotic skin lesions. Similarly, smoking and alcohol consumption were not independently associated with the development of keratotic skin lesions.
In contrast to keratotic skin lesions, verrucae vulgares and plantar warts were more prevalent among younger individuals with a slight but not statistically significant preference for women. Skin phototype did not appear to play a role; also sun-related factors, smoking, and alcohol consumption were not risk factors for the development of verrucae vulgares and plantar warts.
Keratotic skin lesions, viral warts, and skin cancer risk
We confirmed the strong association between the presence and number of keratotic skin lesions and squamous-cell carcinoma in all study centers and at all time points after transplantation. A weaker, but still statistically significant association was found between these lesions and basal-cell carcinoma.
The presence of verrucae vulgares and/or plantar warts was not associated with basal-cell carcinoma. The recipients with squamous-cell carcinoma more often had verrucae vulgares and/or plantar warts, but only in those transplanted 18 years or longer. Misclassification of keratotic skin lesions into verrucae vulgares cannot be completely excluded. Keratotic skin lesions and verrucae vulgares are sometimes difficult to distinguish, especially in the recipients with numerous keratotic skin lesions who are also often those with squamous-cell carcinoma. Misclassification may have led to preferentially higher counts of “verrucae vulgares” in recipients with squamous-cell carcinoma. The fact, however, that we also found a weak association between verrucae vulgares and/or plantar warts localized only on the palms and soles, which are sites on the body where confusion with keratotic skin lesions is much less likely, provides support for the argument that verrucae vulgares and/or plantar warts may be associated with risk of squamous-cell carcinoma. It is probable that the presence of persistent palmar/plantar viral warts in patients with squamous-cell carcinoma reflects the degree to which these long-term transplant recipients are immunosuppressed.
Sun exposure, level of education, and skin cancer risk
As expected, painful sunburns before the age of 20 years were associated with an increased risk of both squamous- and basal-cell carcinoma. However, we did not find a clear association between chronic sun exposure and skin cancer. Recall bias, especially in the recipients with skin cancer or confusion between sun exposure before and after the transplantation may be possible factors explaining the absence of association between chronic sun exposure and skin cancer. The investigators were instructed in all centers to ask for chronic sun exposure before transplantation. Nevertheless, in some countries sun exposure before and after transplantation may have been incorrectly recalled, particularly in long-term recipients. In addition, there may have been variations between the centers in timing and consistency of advice provided on photo protection and it is likely that reinforcement of such advice may have been more rigorously emphasized in patients with skin cancer. It should also be realized that the relative intensity of UV differs with changing geographical latitude.
We found an association between education and basal-cell, but not squamous-cell carcinoma. It has been hypothesized that basal-cell carcinoma is related to intermittent, rather than chronic sun exposure (Armstrong and Kricker, 2001), which is likely to occur more frequently among those with higher socio-economic status who have the leisure time and money to engage in sunny holidays and recreational pursuits. Socio-economic status may be a more reliable tool to measure indirectly intermittent sun exposure than the direct question about sun exposure at weekends, because we were not able to show an association between the latter factor and basal-cell carcinoma.
Smoking or alcohol and skin cancer
We did not find consistent associations between smoking or alcohol consumption and skin cancer. Exposures to tobacco and alcohol were quite different in the five countries, with the lowest exposure in Germany and the highest in France. In the immunocompetent population, smoking has been implicated as a risk factor for squamous-cell carcinoma (De Hertog et al., 2001; Freedman et al., 2003; Rosenquist et al., 2005). In The Netherlands and Germany the same trend was observed, but in the United Kingdom and France a trend toward a negative association between smoking and squamous-cell carcinoma was observed.
Similarly, alcohol consumption was positively associated with squamous-cell carcinoma in The Netherlands, United Kingdom, and Germany and negatively in France. The organ-transplant recipient populations in the five countries may have been too heterogeneous with respect to smoking and alcohol consumption for any useful conclusions to be drawn about the association between these factors and skin cancer in this patient population. However, the data concerning the association between alcohol consumption and skin cancer are intriguing, and justify further investigation (Freedman et al., 2003; Rosenquist et al., 2005).
Immunosuppression and skin cancer
Immunosuppression with azathioprine has been linked to an increased risk of skin cancer possibly through selective UVA photosensitivity (O'Donovan et al., 2005). In our study we did not find a significantly increased risk, possibly because the immunosuppressive regimens differed much between the different centers and types of organs transplanted to allow definitive comparisons to be drawn.
Is there indirect evidence of a role for beta-papillomaviruses in transplant skin cancers?
The strong association with keratotic skin lesions provides indirect evidence of a role for beta-papillomaviruses in the etiology of cutaneous squamous-cell carcinoma (Boyle et al., 1984; Shuttleworth et al., 1987; Blohme and Larko, 1990; Bouwes Bavinck et al., 1993; de Jong-Tieben et al., 2000) and possibly also basal-cell carcinoma. Earlier studies have shown beta-papillomavirus DNA in squamous-cell carcinomas and the associated premalignant lesions (de Jong-Tieben et al., 1995, 2000; Harwood et al., 1999, 2000; Berkhout et al., 2000; Pfister et al., 2003; Weissenborn et al., 2005). In contrast, beta-papillomavirus types are not prevalent in common or palmoplantar warts.
An alternative hypothesis is that immunosuppression leads to the development of keratotic skin lesions and skin cancer independently, and our data cannot exclude this possibility. One argument against this might be that development of keratotic skin lesions precedes that of skin cancer by several years (de Jong-Tieben et al., 2000), but further studies are required to prove that beta-papillomavirus infection plays a causal role in the development of skin cancer.
Practical implications of these data: predictive risk factors for skin cancer
The number of keratotic skin lesions is, by far, the most objective clinical criterion predictive of an increased risk of squamous- and basal-cell carcinoma in organ-transplant recipients. Age and time period after transplantation are other useful criteria to estimate the risk of skin cancer. Assessment of cumulative sun exposure, smoking, and alcohol consumption are, in our opinion, too subjective to be used for screening purposes, and there are insufficient data relating to the nature of the immunosuppressive regimen to use this as a predictor of skin cancer risk.
In conclusion, the high numbers of keratotic skin lesions following transplantation and their strong association with skin cancer risk provide support for a causative role for beta-papillomavirus infection in the development of post-transplant squamous-cell carcinoma. Of particular importance to management, numbers of keratotic skin lesions are an easily assessable clinical parameter for identifying organ-transplant recipients, who are at an increased risk for skin cancers and may require more intensive surveillance in specialized organ-transplant skin clinics.
MATERIALS AND METHODS
Study population
A hospital-based case–control study was designed to assess possible risk factors for skin cancer and keratotic skin lesions in organ-transplant recipients who had been transplanted at least 2 years previously. Patients with solid organ transplants were recruited in the following hospitals: Leiden University Medical Center, Leiden, The Netherlands; Bart's and the London NHS Trust, London, UK; University Clinic Charité, Berlin, Germany; Hôpital Edouard Herriot, Lyon, France; Ospedali Riuniti di Bergamo, Bergamo, Italy; and Ospedale Civile Maggiore, Verona, Italy.
Cases were defined as patients with a current skin cancer (squamous- and basal-cell carcinoma) and a skin cancer in their medical history. Cases with skin cancer were selected from the outpatient nephrology and dermatology clinics and controls (without a history of skin cancer) were selected from the same outpatient clinics. We attempted to frequency-match for sex, age group (−5 years to +20 years), and time since the first transplantation (2–7, 8–12, 13–17, 18–22, and 23 and more years after transplantation). Patients with (Fitzpatrick) skin type V and VI were excluded from the study. The study adhered to the Declaration of Helsinki Principles and the local medical ethical committees of the hospitals in the five countries had approved the study design. Participants gave their written informed consent.
Collection of data
Questionnaires and medical charts were used to gather the following information: sex and age of the patients; type and number of solid organ transplantations; level of education; UV-related questions, such as ability to tan, sun reactivity (Fitzpatrick), skin type, occupational sun exposure (during the week), recreational sun exposure during the weekends, and number of painful sunburns before the age of 20 years; and other potential risk factors for skin cancer such as smoking (non-smokers, ex-, and current smokers; number of cigarettes and duration of smoking) and alcohol consumption during the week and weekend.
Skin type (skin phototype) was re-categorized into “olive”, “medium”, and “fair” depending on responses to the questions about tanning ability, sun reactivity, and Fitzpatrick skin type (Figure S1).
Occupational sun exposure in adulthood was ascertained for the period before the first transplantation and was dichotomized into low (<4 hours/day) and high (⩾4 hours/day). Recreational sun exposure during the weekends was similarly dichotomized. The number of painful sunburns was collected as no sunburns, 1–4 sunburns, or 5 and more sunburns before the age of 20 years.
Data about alcohol consumption during the week were collected for the periods between Monday and Friday morning and during the weekend from Friday evening to Sunday evening. Alcohol consumption was divided into no consumption, low consumption (1–19 g/day), and high consumption (⩾20 g/day) with the help of a list indicating the amount of alcohol per drink.
The number and type of all skin cancers were collected for the cases before and after the first transplantation. Only histologically proven skin cancers were included in the study analyses. The skin was examined according to a set protocol in both cases and controls. Biopsies were taken for histological confirmation of any lesions clinically suspicious for skin cancer.
At a consensus meeting all clinical investigators agreed on the definition of warts and keratotic skin lesions to ensure validity across the five centers. Verrucae vulgares were defined as hyperkeratotic, exophytic and dome-shaped papules, or nodules with punctuate black dots on the hyperkeratotic surface. Plantar (and palmar) warts were defined as thick, endophytic papules on the soles and palms. All other hyperkeratotic skin lesions were counted as “keratotic skin lesions”, consisting of actinic keratoses, seborrheic warts, flat warts, and hyperkeratotic papillomas. The keratotic skin lesions and the verrucae vulgares and/or plantar (and palmar) warts were counted separately on the face, chest, arms and dorsum of the hands, legs and dorsum of the feet, and on the palms and soles.
We combined actinic keratoses, seborrheic warts, plane warts, and hyperkeratotic papillomas during the counting procedure, because it is often difficult, time-consuming, and sometimes impossible to separate these lesions on clinical grounds. For practical reasons, we also combined common warts and palmoplantar warts during the counting procedure. It was not feasible to perform systematic histological diagnosis of keratotic skin lesions, common, and palmoplantar warts in this study.
Statistical analyses
All analyses were performed with SPSS version 12 for Windows. χ2 tests were used to compare differences in categorical variables between cases and controls or between different countries or other characteristics.
Relative risks of developing skin cancer, keratotic skin lesions, or verrucae vulgares and/or plantar warts were estimated using exposure ORs from cross-tabulation and logistic regression. As cases and controls were frequency-matched rather than individually matched we did not use conditional logistic regression, but adjusted for all matching factors in the analysis. Thus ORs were adjusted for age, sex, years after transplantation and, when all countries were taken together, also for study center. Unless otherwise specified, future reference to adjustment refers to adjustment for these factors.
For analyses of squamous-cell carcinomas, we did not exclude patients also with basal-cell carcinomas. Restricting the analyses to patients with squamous-cell carcinomas with no history of basal-cell carcinoma; however, did not result in substantially different outcomes. For the analyses with basal-cell carcinomas as the outcome, we included patients with basal-cell carcinomas only with no history of squamous-cell carcinoma.
Altogether 91 of the 937 recipients had one or more histologically confirmed Bowen's disease (carcinoma –in situ) (22 cases in the recipients without skin cancer and 69 cases in recipients with skin cancer). The 22 recipients without skin cancer who had Bowen's disease were excluded from all analyses, because these lesions closely resemble squamous-cell carcinomas.
Eleven patients were recruited who had undergone their organ transplant less then 2 years previously (two controls in the United Kingdom, and seven controls and two patients in Germany: one with squamous-cell carcinoma and one with basal-cell carcinoma). As excluding these made no difference to the results, they were included in the 2–7 years post-transplant category.
Because of missing values for some variables the groups to be analyzed varied between 909 and 915 recipients.
ACKNOWLEDGMENTS
Financial support from the European Commission for this study (QLK2-CT-2002-0117) is greatly acknowledged. Rachel Neale is supported by a National Health and Medical Research Council (Australia) Sidney Sax Postdoctoral Fellowship. Catherine Harwood and Charlotte Proby are supported by Cancer Research-UK, and grant RAC404 from the research advisory board of St Bartholomew's and the Royal London Charitable Foundation. Damiano Abeni and Francesca Sampogna are also supported, in part, by the ‘Progetto Ricerca Corrente’ of the Italian Ministry of Health. Gianpaolo Tessari was given a grant from the Banca Popolare di Verona, Verona Italy. Mariet Feltkamp is supported by The Netherlands Organisation for Health Research and Development, Clinical Fellowship grant 907-00-150.
APPENDIX
Members of the EPI-HPV-UV-CA group are: Department of Dermatology, Leiden University Medical Center, Leiden, The Netherlands: J.N. Bouwes Bavinck, P. van der Zwan-Kralt, Y.G.L. de Graaf, L.E. Vos, E.J. Uphoff-Meijerink and R. Willemze. Department of Medical Microbiology, Leiden University Medical Center, Leiden, The Netherlands: M.C.W. Feltkamp, L. Struijk, P. Wanningen, P.Z. van der Meijden and E.I. Plasmeijer. Department of Medical Statistics, Leiden University Medical Center, Leiden, The Netherlands: R. Wolterbeek. Department of Dermatology, Hospital Edouard Herriot, Lyon, France: S. Euvrard, A.C. Butnaru, A. Claudy and J. Kanitakis. Department of Dermatology, University Hospital Charité, Skin Cancer Center Charité, Berlin, Germany: I. Nindl, E. Stockfleth and T. Forschner. Department of Dermatology, Ospedali Riuniti, Bergamo, Italy: L. Naldi, A. Pizzagalli and F. Sassi. Department of Biomedical and Surgical Sciences, Section of Dermatology, University of Verona, c/o Ospedale Civile Maggiore, Verona Italy: G. Tessari. Centre for Cutaneous Research, Institute of Cell and Molecular Science, Bart's and the London, Queen Mary's School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom: C.A. Harwood, C.M. Proby, J. Breuer, L. Mitchell, K. Purdie, S.R. Lambert and H. Ran. Institute of Virology, University of Cologne, Cologne, Germany: H. Pfister, U. Wieland and S. Weissenborn. German Cancer Research Center (DKFZ), Heidelberg, Germany: M. Pawlita, T. Waterboer, P. Sehr and K. Michael. DDL Diagnostic Laboratory, Voorburg, The Netherlands: W.G.V. Quint, M.N.C. de Koning*, J. ter Schegget*, B. Kleter and L.J. van Doorn. *Also employed by Department of Medical Microbiology, Leiden University Medical Center, Leiden, The Netherlands Istituto Dermopatico dell'Immacolata, IDI-IRCCS, Rome, Italy: D. Abeni, F. Sampogna, T.J. Mannooranparampil, N. Melo-Salcedo, S. Simoni, G.P. Petasecca Donati, C. Masini, and C. Deppermann Fortes. Queensland Institute of Medical Research and Queensland Cancer Fund, Brisbane Australia: A.C. Green, R. Neale and C. Olsen. James Cook University; S. Harrison and P. Buttner.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Adami J, Gabel H, Lindelof B, Ekstrom K, Rydh B, Glimelius B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–7. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- Berg D, Otley CC. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1–17. doi: 10.1067/mjd.2002.125579. [DOI] [PubMed] [Google Scholar]
- Berkhout RJ, Bouwes Bavinck JN, ter Schegget J. Persistence of human papillomavirus DNA in benign and (pre)malignant skin lesions from renal transplant recipients. J Clin Microbiol. 2000;38:2087–96. doi: 10.1128/jcm.38.6.2087-2096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blohme I, Larko O. Skin lesions in renal transplant patients after 10–23 years of immunosuppressive therapy. Acta Dermatol Venereol. 1990;70:491–4. [PubMed] [Google Scholar]
- Bouwes Bavinck JN, De Boer A, Vermeer BJ, Hartevelt MM, van der Woude FJ, Claas FH, et al. Sunlight, keratotic skin lesions and skin cancer in renal transplant recipients. Br J Dermatol. 1993;129:242–9. doi: 10.1111/j.1365-2133.1993.tb11841.x. [DOI] [PubMed] [Google Scholar]
- Bouwes Bavinck JN, Feltkamp MC. Milk of human kindness? – HAMLET, human papillomavirus, and warts. N Engl J Med. 2004;350:2639–42. doi: 10.1056/NEJMp048086. [DOI] [PubMed] [Google Scholar]
- Bouwes Bavinck JN, Hardie DR, Green A, Cutmore S, MacNaught A, O'Sullivan B, et al. The risk of skin cancer in renal transplant recipients in Queensland, Australia. A follow-up study. Transplantation. 1996;61:715–21. doi: 10.1097/00007890-199603150-00008. [DOI] [PubMed] [Google Scholar]
- Bouwes Bavinck JN, Vermeer BJ, van der Woude FJ, Vandenbroucke JP, Schreuder GM, Thorogood J, et al. Relation between skin cancer and HLA antigens in renal-transplant recipients. N Engl J Med. 1991;325:843–8. doi: 10.1056/NEJM199109193251203. [DOI] [PubMed] [Google Scholar]
- Boyle J, MacKie RM, Briggs JD, Junor BJ, Aitchison TC. Cancer, warts, and sunshine in renal transplant patients. A case–control study. Lancet. 1984;1:702–5. doi: 10.1016/s0140-6736(84)92221-9. [DOI] [PubMed] [Google Scholar]
- De Hertog SA, Wensveen CA, Bastiaens MT, Kielich CJ, Berkhout MJ, Westendorp RG, et al. Relation between smoking and skin cancer. J Clin Oncol. 2001;19:231–8. doi: 10.1200/JCO.2001.19.1.231. [DOI] [PubMed] [Google Scholar]
- de Jong-Tieben LM, Berkhout RJ, Smits HL, Bouwes Bavinck JN, Vermeer BJ, van der Woude FJ, ter Schegget J. High frequency of detection of epidermodysplasia verruciformis-associated human papillomavirus DNA in biopsies from malignant and premalignant skin lesions from renal transplant recipients. J Invest Dermatol. 1995;105:367–71. doi: 10.1111/1523-1747.ep12320803. [DOI] [PubMed] [Google Scholar]
- de Jong-Tieben LM, Berkhout RJ, ter Schegget J, Vermeer BJ, de Fijter JW, Bruijn JA, et al. The prevalence of human papillomavirus DNA in benign keratotic skin lesions of renal transplant recipients with and without a history of skin cancer is equally high: a clinical study to assess risk factors for keratotic skin lesions and skin cancer. Transplantation. 2000;69:44–59. [PubMed] [Google Scholar]
- Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- Feltkamp MC, Broer R, di Summa FM, Struijk L, van der ME, Verlaan BP, et al. Seroreactivity to epidermodysplasia verruciformis-related human papillomavirus types is associated with nonmelanoma skin cancer. Cancer Res. 2003;63:2695–700. [PubMed] [Google Scholar]
- Fortina AB, Piaserico S, Caforio AL, Abeni D, Alaibac M, Angelini A, et al. Immunosuppressive level and other risk factors for basal cell carcinoma and squamous cell carcinoma in heart transplant recipients. Arch Dermatol. 2004;140:1079–85. doi: 10.1001/archderm.140.9.1079. [DOI] [PubMed] [Google Scholar]
- Freedman DM, Sigurdson A, Doody MM, Mabuchi K, Linet MS. Risk of basal cell carcinoma in relation to alcohol intake and smoking. Cancer Epidemiol Biomarkers Prev. 2003;12:1540–3. [PubMed] [Google Scholar]
- Hartevelt MM, Bouwes Bavinck JN, Kootte AM, Vermeer BJ, Vandenbroucke JP. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation. 1990;49:506–9. doi: 10.1097/00007890-199003000-00006. [DOI] [PubMed] [Google Scholar]
- Harwood CA, Spink PJ, Surentheran T, Leigh IM, de Villiers EM, McGregor JM, et al. Degenerate and nested PCR: a highly sensitive and specific method for detection of human papillomavirus infection in cutaneous warts. J Clin Microbiol. 1999;37:3545–55. doi: 10.1128/jcm.37.11.3545-3555.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood CA, Surentheran T, McGregor JM, Spink PJ, Leigh IM, Breuer J, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289–97. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Iftner T, Elbel M, Schopp B, Hiller T, Loizou JI, Caldecott KW, et al. Interference of papillomavirus E6 protein with single-strand break repair by interaction with XRCC1. EMBO J. 2002;21:4741–8. doi: 10.1093/emboj/cdf443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 2000;14:3065–73. doi: 10.1101/gad.182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P, Moller B, Hansen S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 2000;42:307. doi: 10.1016/s0190-9622(00)90154-3. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Nelson HH, Sehr P, Waterboer T, Stukel TA, Andrew A, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389–95. doi: 10.1093/jnci/djj092. [DOI] [PubMed] [Google Scholar]
- Lindelof B, Granath F, Dal H, Brandberg Y, Adami J, Ullen H. Sun habits in kidney transplant recipients with skin cancer: a case-control study of possible causative factors. Acta Dermatol Venereol. 2003;83:189–93. doi: 10.1080/00015550310007193. [DOI] [PubMed] [Google Scholar]
- Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513–9. [PubMed] [Google Scholar]
- Masini C, Fuchs PG, Gabrielli F, Stark S, Sera F, Ploner M, et al. Evidence for the association of human papillomavirus infection and cutaneous squamous cell carcinoma in immunocompetent individuals. Arch Dermatol. 2003;139:890–4. doi: 10.1001/archderm.139.7.890. [DOI] [PubMed] [Google Scholar]
- Moloney FJ, Comber H, O'Lorcain P, O'Kelly P, Conlon PJ, Murphy GM. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol. 2006;154:498–504. doi: 10.1111/j.1365-2133.2005.07021.x. [DOI] [PubMed] [Google Scholar]
- Naldi L, Fortina AB, Lovati S, Barba A, Gotti E, Tessari G, et al. Risk of nonmelanoma skin cancer in Italian organ transplant recipients. A registry-based study. Transplantation. 2000;70:1479–84. doi: 10.1097/00007890-200011270-00015. [DOI] [PubMed] [Google Scholar]
- O'Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309:1871–4. doi: 10.1126/science.1114233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H, Fuchs PG, Majewski S, Jablonska S, Pniewska I, Malejczyk M. High prevalence of epidermodysplasia verruciformis-associated human papillomavirus DNA in actinic keratoses of the immunocompetent population. Arch Dermatol Res. 2003;295:273–9. doi: 10.1007/s00403-003-0435-2. [DOI] [PubMed] [Google Scholar]
- Ramsay HM, Fryer AA, Hawley CM, Smith AG, Harden PN. Nonmelanoma skin cancer risk in the Queensland renal transplant population. Br J Dermatol. 2002;147:950–6. doi: 10.1046/j.1365-2133.2002.04976.x. [DOI] [PubMed] [Google Scholar]
- Ramsay HM, Fryer AA, Reece S, Smith AG, Harden PN. Clinical risk factors associated with nonmelanoma skin cancer in renal transplant recipients. Am J Kidney Dis. 2000;36:167–76. doi: 10.1053/ajkd.2000.8290. [DOI] [PubMed] [Google Scholar]
- Rosenquist K, Wennerberg J, Schildt EB, Bladstrom A, Hansson BG, Andersson G. Use of Swedish moist snuff, smoking and alcohol consumption in the aetiology of oral and oropharyngeal squamous cell carcinoma. A population-based case–control study in southern Sweden. Acta Otolaryngol. 2005;125:991–8. doi: 10.1080/00016480510043440. [DOI] [PubMed] [Google Scholar]
- Shuttleworth D, Marks R, Griffin PJ, Salaman JR. Dysplastic epidermal change in immunosuppressed patients with renal transplants. Q J Med. 1987;64:609–16. [PubMed] [Google Scholar]
- Struijk L, Bouwes Bavinck JN, Wanningen P, van der ME, Westendorp RG, ter Schegget J, et al. Presence of human papillomavirus DNA in plucked eyebrow hairs is associated with a history of cutaneous squamous cell carcinoma. J Invest Dermatol. 2003;121:1531–5. doi: 10.1046/j.1523-1747.2003.12632.x. [DOI] [PubMed] [Google Scholar]
- Vallejo GH, Romero CJ, de Vicente JC. Incidence and risk factors for cancer after liver transplantation. Crit Rev Oncol Hematol. 2005;56:87–99. doi: 10.1016/j.critrevonc.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Weissenborn SJ, Nindl I, Purdie K, Harwood C, Proby C, Breuer J, et al. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Invest Dermatol. 2005;125:93–7. doi: 10.1111/j.0022-202X.2005.23733.x. [DOI] [PubMed] [Google Scholar]


