Abstract
Background
Antenatal clinic (ANC) surveillance is the primary source of HIV prevalence estimates in low-resource settings. In younger women prevalence approximates to incidence. Sexual behaviour monitoring to explain HIV distribution and trends is seldom attempted in ANC surveys. We explore the use of marital history in ANC as a proxy for sexual behaviour.
Methods
Five ANC clinics in a rural African district participated in surveillance from 1999-2004. Unlinked anonymous HIV testing and marital history interviews (including age at first sex and socio-economic variables) were conducted. Data on women aged less than 25 were analysed.
Results
Inferred sexual exposure pre-marriage and post-first marriage increased the adjusted odds of infection with HIV by more than 0.1 for each year of exposure. Increasing years within a first marriage did not increase HIV risk. After adjusting for age, women in more recent birth cohorts were less likely to be infected.
Conclusions
Marital status is useful behavioural information and can be collected in ANC surveys. Exposure in an ongoing first marriage did not increase the odds of infection with HIV in this age group. HIV prevalence decreased over time in young women. ANC surveillance programmes should develop proxy sexual behaviour questions, particularly in younger women.
Keywords: ante-natal clinic, surveillance, HIV, sexual behaviour, Malawi
Background
After rapid increases in the 1980s and early 1990s, HIV prevalence has recently stabilized or declined in several high prevalence countries in sub-Saharan Africa[1, 2]. There is need to document and explain trends in HIV prevalence[3-5] and to relate the changes to behaviour, particularly when trends are inconsistent between countries and population groups, and to establish whether changes in prevalence are accompanied by behaviour change[5]. “Second generation” surveillance aims to explain as well as describe the changes in HIV prevalence[3, 6] in order to inform policy directly of changes in risk behaviour.
To date, antenatal clinic (ANC) surveillance has been the main source of HIV prevalence trend data. It has not been used for sexual behaviour monitoring as detailed inquiry has been considered time-consuming and intrusive in the context of unlinked anonymous surveillance within an essential clinical service[7], despite the fact that it is important to examine sexual behaviour in the same population from which HIV prevalence data are collected. Much effort has recently gone into designing improved sampling frames for anonymous ANC surveillance in order to ensure wide coverage, and to minimise self-selection in multiple-provider settings or when services such as Prevention of Mother to Child Transmission programmes (PMTCT) offer named testing. It is therefore important to maximise the usefulness of data collected.
Marital history may be a good proxy for sexual behaviour insofar as it allows identification of periods of varying exposure to HIV: eg relatively low risk periods (stable first marriage) and higher risk periods (pre-marital exposure, marriages that break up, re-marriages).
Amongst women aged less than 25, HIV prevalence is a reasonable proxy for incidence, as infections are relatively recent and HIV-related mortality relatively low. Marital stability in this age group is also a sensitive indicator of community behaviour change as an appreciable proportion of the group is replenished each year by newly sexually active entrants.
This paper explores the use of ANC surveillance to infer behaviours relevant to HIV transmission in rural northern Malawi, where the Karonga Prevention Study (KPS) has been conducting epidemiological studies of HIV since the 1980s[8]. Since 1999 these studies included ANC sentinel surveillance in five health facilities in Karonga district, using a variant of Malawi's national data collection protocol[9]. Data presented here demonstrate the utility of ANC surveillance in addressing the relationship between sexual behaviour patterns and observed trends in HIV prevalence.
Methods
Between 1999 and 2004, ANC unlinked anonymous surveillance was conducted in five main health facilities: one district hospital [urban], two rural hospitals [one semi-urban, one rural] and two health centres [rural] of Karonga district, northern Malawi. From 2003, data collection ceased in clinics one by one due to the introduction of PMTCT which complicated anonymous data collection.
Questionnaires were anonymised and dual HIV testing was carried out using ELISA and particle agglutination assays [9]. Data collection included standard questions, age at start of current marriage, age at start of first marriage and (from 2002 onwards) age at first sex. “Marriage” was defined by the respondent and in this population usually refers to a union following a traditional ceremony or a community-acknowledged state of co-habitation. “First sex” was defined as first penetrative sex; and the interviewers (who were all female, with study-specific training and completed primary education) were trained to ensure that this was correctly established in the vernacular. Interviews were carried out in private.
Approval for the study was granted by the Malawi National Health Sciences Research Committee and the Ethics Committee of the London School of Hygiene and Tropical Medicine.
Data were double entered and verified in FoxPro and managed and analysed in Stata (version 8.1). Women were grouped for analysis by area of current residence, having been coded by village-groups to avoid inadvertently identifying individuals.
Years spent in each of three separate “sexual exposure” categories were calculated for each woman. Duration of pre-marital exposure was calculated as [current age – age at first sex] for never married and [age at first marriage – age at first sex] for those ever married; representing a period when a woman may have multiple, unstable partnerships. Duration of first marriage exposure was calculated as [current age – age at first marriage] for those still in their first marriage, and for those for whom their first marriage had ended as [age at end of first marriage – age at first marriage], and represents a period where sexual exposure is likely to be confined to a single partner and to be related to child bearing. Marital status questions identified women whose first marriage had ended. However, age at end of first marriage was not recorded so this was estimated by allowing 2 years of marriage for every child born in that marriage, or, when no children, allocation to the marriage 2/3 of the interval between the start of the first marriage and the start of a second marriage and the remaining 1/3 to an inter-marriage period or 4/5 of the interval between start of first marriage and current day for those who had not re-married (based on observations from large demographic surveillance studies at KPS, which link children and spousal relationships)[10]. Time after first marriage included all other periods since sexual debut. This is another period that may include multiple sexual partners and may involve economic dependence on sexual partners, once away from the parental home. For the calculation of rates, the time after the first marriage was divided further into re-married and ex-married periods, according to whether the time spent after the first marriage was spent married or single.
Information on age at first sex was incomplete, as the question was not included in the first version of the ANC questionnaire (1999-2001). However, upper bounds for the start of sexual activity have been established for those with missing or inconsistent information, as exposure to sexual activity must have occurred at least half a year prior to the ANC visit for women expecting their first birth [current age – 0.5], and at least nine months before first birth for those who had previously given birth [age at first birth (AAFB) – 0.75]. For married women, sexual activity can be presumed to start at or before first marriage, and time spent in pre-marital sexual activity was estimated for missing data using predicted median pre-marital exposure based on age at first sex to age at first marriage using a Poisson regression method. Thus
The HIV prevalence in ANC women aged 25 and over in each residence area was used as a proxy for background HIV prevalence in the population from which the young women drew their partners[4, 11].
Logistic regression of HIV status was undertaken against continuous measures of exposure (e.g., time spent at risk and background HIV prevalence) and standard categorical and binary variables (e.g. current marital status and parity; behavioural and health indicators such as past family-planning use, past experience of child mortality, and socio-demographic variables such as education, area of residence, and age of father of the child).
A classification of “low parity for age” was devised to include women in the top quartile of the age range for a given parity; for the study group of interest this comprised those aged 20 or over who were expecting their first birth .
Results
Less than 1% of women seen at the ANC clinics refused interview or venepuncture. ANC data (interview and serology) were available on 2,874 women under 25 over the six year period. This excluded 13 women resident outside the district, one woman with missing birth year and 44 ever-married women with missing age at marriage. Age at marriage data were internally consistent with other age-related events, such as birth of children within marriage, for all but five women, who were excluded. “Age at first sex” was not available for 1679 women interviewed between 1999-2001; and was missing (or inconsistent) for 547 of 1195 women interviewed in 2002-04. Those not reporting age at first sex when questioned directly were mainly (60%) married women, over 20, with recall difficulty. Table 1 shows the age distribution and mean years of sexual exposure at each age, comparing the mean duration of sexual exposure among those for whom age at first sex was imputed and those for whom it was measured directly. Data for earlier and later periods are presented separately to reveal period factors affecting imputed ages at first sex. Only 21 women aged 18 and under, reporting in 2002-04, had imputed age at first sex.
Table 1. Number of women aged <25 contributing to Antenatal Surveillance by current age, with mean years of sexual exposure, by age and estimation method of age at sexual debut.
| When tested | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1999-2001 |
2002-2004 |
All years |
||||||
| Age | Imputed (for all) |
Reported (excluding misreports)** |
Imputed (non- responders and misreports)** |
Total | Reported | Imputed | Total | Total number of women |
| 15 | 1.13 | 0.88 | 0.88 | 0.88 | 1.13 | 1.04 | 49* | |
| 16 | 1.45 | 1.02 | 2.00 | 1.09 | 1.02 | 1.47 | 1.32 | 118 |
| 17 | 1.63 | 1.63 | 2.33 | 1.73 | 1.63 | 1.70 | 1.67 | 306 |
| 18 | 1.97 | 1.98 | 2.89 | 2.16 | 1.98 | 2.10 | 2.06 | 405 |
| 19 | 2.98 | 2.77 | 3.01 | 2.87 | 2.77 | 2.99 | 2.94 | 423 |
| 20 | 3.68 | 3.43 | 3.70 | 3.56 | 3.43 | 3.69 | 3.63 | 389 |
| 21 | 4.51 | 4.47 | 4.32 | 4.38 | 4.47 | 4.45 | 4.45 | 349 |
| 22 | 5.40 | 5.08 | 5.46 | 5.34 | 5.08 | 5.42 | 5.38 | 296 |
| 23 | 6.39 | 6.40 | 6.26 | 6.30 | 6.40 | 6.35 | 6.36 | 267 |
| 24 | 7.43 | 7.63 | 7.01 | 7.17 | 7.63 | 7.29 | 7.33 | 272 |
| All ages | 3.87 | 3.03 | 4.71 | 3.80 | 3.03 | 4.08 | 3.84 | |
| Number of women |
1,679 | 648 | 547 | 1,195 | 648 | 2,226 | 2,874 | 2,874 |
includes two 13 year old and nine 14 year old girls.
76 misreports comprise: age at first sex later than current age (5); later than age at first marriage (14); later than or same as age at first birth (55)
Over the time period examined (1999-2004), the age distribution of those under 25 at interview did not change (mean = 20.0 years) nor did mean ages at first marriage (17.5) or first birth (18.1).
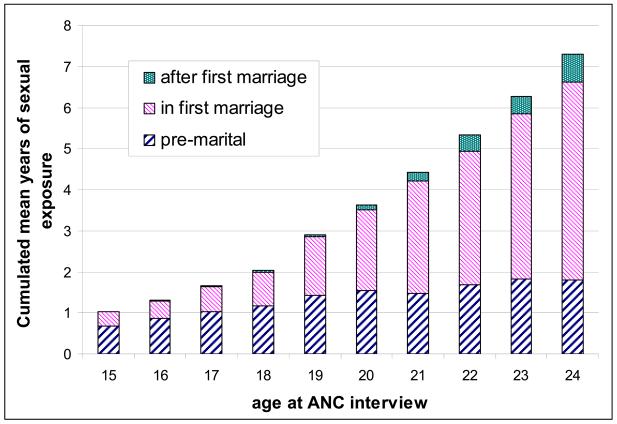
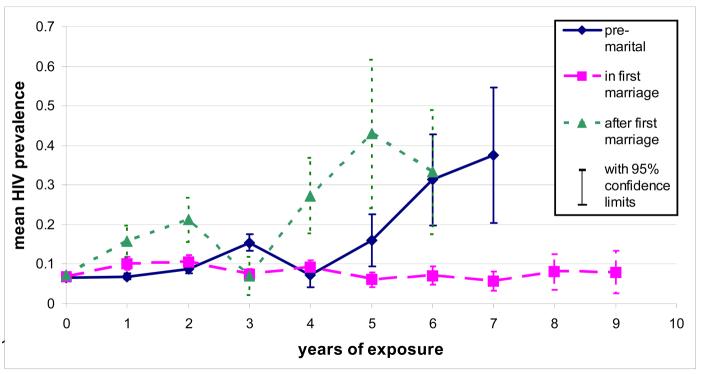
The distribution of mean time spent in each sexual exposure category for women aged under 25 years at interview showed a plausible pattern with age (Figure 1). Pre-marital exposure was similar across all ages, with a mean of just over one year; exposure after end of first marriage only becomes important after 20 years of age. Unadjusted HIV prevalence is shown in Figure 2 by duration in each “sexual exposure” category. There is a progressive increase in HIV risk associated with longer durations (more than 4 years) of pre-marital exposure, and longer duration since first marriage ended. Increasing duration of sexual exposure in a first marriage was not associated with increased risk of HIV infection in this age group.
Figure 1.
Mean person-years lived in different marital status categories by current age, for women under 25 at ANC interview
Figure 2.
Mean HIV prevalence by years of exposure in different marital status categories, for women under 25 at ANC interview
To refine the estimates of the effect of marriage status, other risk factors were examined for women under 25. Crude HIV prevalence and odds of HIV infection are shown in Table 2. Increasing age, area of residence, current marital status, birth cohort, parity for age, experience of child death, age of sexual partner, evidence of syphilis, previous use of contraception, previous residence outside the district and secondary education were all significantly associated with HIV infection.
Table 2. Distribution of women under 25 contributing to Antenatal Surveillance by risk category, with HIV prevalence and crude odds ratios for infection.
| Risk categories | N | % | % HIV+ | OR | 95% CI | |
|---|---|---|---|---|---|---|
| total women | 2,874 | 100.0 | 8.0 | |||
| age at ANC attendance | ||||||
| <16 | 167 | 5.8 | 3.0 | 0.42 | 0.16-1.06 | |
| 17-18 | 711 | 24.7 | 4.9 | 0.70 | 0.45-1.08 | |
| 19-20 | 812 | 28.2 | 6.9 | 1 | ||
| 21-22 | 645 | 22.4 | 10.4 | 1.56 | 1.08-2.27 | |
| 23-24 | 539 | 18.8 | 12.6 | 1.95 | 1.34-2.83 | |
| area of current residence | ||||||
| rural / truckstop | 462 | 16.1 | 7.8 | 1.44 | 0.91-2.27 | |
| rural mid-district | 774 | 26.9 | 5.6 | 1 | ||
| peri-urban (township vicinity) | 284 | 9.9 | 8.8 | 1.64 | 0.98-2.74 | |
| urban (township) | 416 | 14.5 | 13.2 | 2.59 | 1.70-3.94 | |
| rural north | 653 | 22.7 | 6.9 | 1.26 | 0.82-1.94 | |
| rural hills and national border | 285 | 9.9 | 9.5 | 1.78 | 1.08-2.94 | |
| current marital status | ||||||
| never married | 118 | 4.1 | 8.5 | 1.25 | 0.64-2.43 | |
| first marriage | 2,474 | 86.1 | 6.9 | 1 | ||
| re-married | 218 | 7.6 | 17.4 | 2.84 | 1.94-4.17 | |
| ex-married | 64 | 2.2 | 18.8 | 3.11 | 1.63-5.93 | |
| surveillance period | ||||||
| 1999-2001 | 1,679 | 58.4 | 8.8 | 1 | ||
| 2002-2004 | 1,195 | 41.6 | 6.9 | 0.77 | 0.58-1.02 | |
| birth cohort | ||||||
| 1975-79 | 800 | 27.8 | 11.7 | 1 | ||
| 1980-84 | 1,729 | 60.2 | 7.3 | 0.60 | 0.45-0.79 | |
| 1985-89 | 345 | 12.0 | 2.9 | 0.22 | 0.12-0.44 | |
| current parity | ||||||
| 0 | 1,403 | 48.8 | 7.3 | 1 | ||
| 1 | 948 | 33.0 | 9.4 | 1.31 | 0.97-1.76 | |
| 2 | 437 | 15.2 | 7.6 | 1.03 | 0.69-1.55 | |
| 3+ | 86 | 3.0 | 7.0 | 0.95 | 0.40-2.22 | |
| parity for age classification | ||||||
| normal or high | 2,485 | 86.5 | 7.3 | 1 | ||
| low | 389 | 13.5 | 12.9 | 1.88 | 1.35-2.62 | |
| previous experience of child death | ||||||
| no previous dead children | 2,591 | 90.2 | 7.5 | 1 | ||
| one or more children died | 283 | 9.8 | 12.7 | 1.79 | 1.23-2.62 | |
| age of sexual partner | ||||||
| younger | 36 | 1.3 | 13.9 | 1.69 | 0.96-2.99 | |
| 0-4 years older | 1,184 | 41.2 | 6.3 | 1 | ||
| 5-9 years older | 1,075 | 37.4 | 8.6 | 1.18 | 0.95-1.47 | |
| 10+ years older | 477 | 16.6 | 10.7 | 1.36 | 1.06-1.74 | |
| age not known | 102 | 3.6 | 7.8 | 1.04 | 0.58-1.84 | |
| syphilis serology * | ||||||
| negative | 2,816 | 98.0 | 7.8 | 1 | ||
| positive | 58 | 2.0 | 17.2 | 2.45 | 1.22-4.90 | |
| previous use of contraception | ||||||
| never used | 2,621 | 91.2 | 7.4 | 1 | ||
| used | 253 | 8.8 | 14.2 | 2.06 | 1.41-3.02 | |
| previous residence | ||||||
| Karonga district | 2,589 | 90.1 | 7.1 | 1 | ||
| other part of Malawi | 214 | 7.4 | 15.9 | 2.47 | 1.66-3.67 | |
| foreign country | 71 | 2.5 | 18.3 | 2.93 | 1.58-5.45 | |
| education | ||||||
| none or incomplete primary | 1,669 | 58.1 | 6.7 | 1 | ||
| completed primary | 730 | 25.4 | 8.5 | 1.29 | 0.93-1.78 | |
| some secondary | 475 | 16.5 | 12.0 | 1.90 | 1.35-2.65 | |
Rapid Plasma Reagin confirmed by Treponema pallidum Particle Agglutination
Variables were entered in a full multi-variate model if the odds ratios of at least one exposure category reached statistical significance or if the variables were part of exhaustive classifications where the other categories have statistically significant odds ratios (OR). Where linear trends could be verified using appropriate tests, categorical variables were replaced with their continuous equivalents (e.g. age, years of exposure in different marital status categories, year of birth, year of interview). Age, interview year and year of birth cannot all be included in the full model, because of co-linearity, and year of birth provided a stronger measure of trend (birth cohort effect) than interview year (calendar time).
After allowing for duration of exposure since sexual debut, classified into exclusive components (pre-marital, first marriage and after first marriage), age lost its explanatory power as a measure of exposure. Marital status at time of interview had no additional explanatory value when duration of exposure is controlled for in this way. Low parity and previous child deaths were excluded from the multivariate model in table 3 as these may be consequences rather than risk factors of HIV infection.
Table 3. Regression analysis of risk factors for HIV infection in women under 25 contributing to Antenatal Surveillance.
| Explanatory variables | Crude | Adjusted** | |||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| woman's age | |||||
| * age at surveillance interview | 1.19 | 1.12 - 1.26 | 0.97 | 0.84 - 1.12 | |
| current residence background prevalence | |||||
| * area prevalence in women aged 25+ | 1.07 | 1.04 - 1.09 | 1.04 | 1.01 - 1.07 | |
| years of sexual exposure | |||||
| * total years exposure | 1.13 | 1.07 - 1.19 | |||
| * premarital | 1.34 | 1.20 - 1.50 | 1.19 | 1.01 - 1.40 | |
| * first marriage | 1.06 | 0.99 - 1.13 | 0.98 | 0.88 - 1.08 | |
| *after first marriage | 1.41 | 1.26 - 1.58 | 1.28 | 1.11 - 1.48 | |
| time trends | |||||
| * woman's birth cohort | 0.86 | 0.82 - 0.91 | 0.91 | 0.82 - 0.99 | |
| partner characteristics | |||||
| * age of father of child | 1.05 | 1.03 - 1.08 | 1.04 | 1.01 - 1.06 | |
| sexual health and behaviour | |||||
| syphilis infection | 2.45 | 1.22 - 4.90 | 2.05 | 0.98 - 4.28 | |
| ever used family planning | 2.06 | 1.41 - 3.02 | 1.45 | 0.95 - 2.20 | |
| previous residence out of Karonga | |||||
| from other part of Malawi | 2.47 | 1.66 - 3.67 | 1.84 | 1.21 - 2.81 | |
| from other country | 2.93 | 1.58 - 5.45 | 2.72 | 1.42 - 5.20 | |
| educational attainment | |||||
| complete primary | 1.29 | 0.93 - 1.78 | 1.30 | 0.92 - 1.83 | |
| some secondary | 1.90 | 1.35 - 2.65 | 1.57 | 1.05 - 2.34 | |
continuous variables, ie OR represent ratio change per year, or for prevalence per 1% for reference category for categorical variables see table 2.
Adjusted OR are adjusted for all the other factors in the model
In both the crude and the multivariate adjusted models, area of residence had a strong effect on the odds of HIV infection. This was quantified by explicitly including measures of the background prevalence (assumed to reflect the likelihood that sexual partners had HIV). The multivariate model showed that the odds ratio for HIV infection in an individual woman under 25 increased by 0.04 with each increase of 1% in HIV prevalence among ANC attenders aged 25 and over resident in the same area, after adjusting for all the other factors. The adjusted odds of HIV infection decreased by 0.09 for each one year increase in calendar year of birth. Additional years of premarital or after-first-marriage exposure each added independently 0.2 and 0.3 per year respectively to the odds of infection. After adjustment for other factors, the increased odds of infection associated with each year in a first marriage seen in the crude model was lost. Sensitivity analyses demonstrated that the odds of HIV infection for sexual exposure in and out of marriage, were robust to the assumptions made on time of end of first marriage (not shown).
For each additional year of the unborn child's father's age, the adjusted odds increased by 0.04, and the effects of previous residence outside the district and secondary school education remained after adjustment for other factors.
To examine the effects of the imputed ages at first sex, the model was run using those with reported AAFS alone. Although the regression results in this limited sample were not all statistically significant, the ORs for the smaller sample generally lay within the confidence intervals for the corresponding values in the overall sample. In the full model, adding “imputation of AAFS” as an explanatory variable did not change results for the other variables and was not a statistically significant risk factor in itself.
Discussion
These data and analyses demonstrate a novel use of ANC surveillance data to reveal changes in HIV infection risk associated with marital history in a rural African community at a time when HIV prevalence is stable and may be starting to decline. Inclusion of marital history questions did not appear to affect participation rates adversely (<1% refusals), although some women may have avoided clinics during recruitment periods. The study design did not allow assessment of the impact of different types of questioning on participation rates.
The data show no evidence of continued risk of HIV acquisition for women below 25 years of age in first marriages, but that extended pre-marital exposure, subsequent extra-marital exposure or exposure during subsequent marriages are all associated with increased risk. This is consistent with findings in urban Kenya and Zambia that less than half of HIV in young married women is acquired within the marriage[12] although analyses of those data concluded that early marriage puts young women at higher risk than intermittent contact associated with pre-marital sex[13]. Studies which found that never married women have lower HIV prevalence than married age-mates may not discriminate between virgins and sexually active single women[14].
The lack of ongoing risk in first marriage may be in part an artefact of the cross-sectional study design as introduction of HIV over time may result in sub-fertility or mortality, removing individuals from the sample. However, it is also consistent with the relative sexual exclusivity of a stable first marriage: unfaithfulness, domestic violence associated with alcohol use and other factors associated with HIV risk also precipitate marital break-up.
The insights these data give into sexual behaviour and associated risk of HIV demonstrate the usefulness of simple interviews during ANC surveillance. As with all ANC based surveillance, results are only generalisable to women who conceive. AIDS is likely to be responsible for much of the widowhood in women under 25, thereby lowering conception risks for women with a high probability of being infected. Relative age of current sexual partner, use of contraception and low parity for age are also associated with increased risk. Other community based data from this project reveal that although more than 99% of women attend antenatal clinic when carrying an established pregnancy, approximately one third of all women of childbearing age have not attended ANC in the four years prior to being surveyed[15]. Nearly 90% of the ANC non-attenders have never had a child (the remainder having unusually long birth intervals), and although a third of these are virgins, there are many women with low fertility or whose lifestyles preclude stable relationships and childbearing, who are at relatively high risk of HIV and need to be studied independently[10, 16].
There are data quality limitations for marital history and pre-marital sex questions in ANC interviews, and selective reporting of behaviour favoured by health education messages may be common in this setting. Health education intensifies over time and younger women may feel less inclined to report actual rather than “acceptable” behaviour (e.g. reporting informal relationships resulting in pregnancy as “marriage”). The lack of a question on the date first marriage ended made it necessary to impute time spent in and after the terminated first marriage in divorcees or widows. However, given those caveats, this study presents clear and plausible findings on recent behaviour amongst young, sexually active women in this community.
These data show a downward trend of HIV prevalence over time in women under 25, which remains significant when other factors are controlled. The introduction of PMTCT to the district did not influence this trend as a real effect nor artefactually, as introduction was low key and piece-meal, and data-collection was not conducted in any clinics with established PMTCT services. We looked at two ways of quantifying this trend: first, looking at change in the odds ratio of infection in relation to calendar year of interview; second, looking at the effect of the women's birth cohort (year of birth). The fact that the latter exerts a stronger effect suggests that younger birth cohorts adopt safer sexual behaviour more quickly, even after allowing for their reduced length of exposure to marital and extra-marital sex.
The unmeasured favourable behaviour change could be fewer sexual partners, less frequent contacts with high risk partners, or increased condom use. Direct questions on these behaviours were introduced only in the latter part of the study; thus insufficient data have been collected to allow for a more complete analysis. Although these questions were completed, interviewers reported an element of reluctance or concealment in answering these direct enquiries as indeed there may have been when reporting age at first sex, although this was not pronounced.
The difficulty in elucidating reliable responses to specific sexual behaviour questions in this high through-put clinical setting, emphasises the value of informative but less controversial questions such as marital history and age at sexual debut, which may be less prone to social desirability biases, and easier to check. Quality of data particularly on the latter question will be improved with appropriate training of interviewers in language and attitudes, by ensuring privacy during interviews and by emphasising confidentiality and anonymity to the client.
In this population, pre-marital sex is the norm and pregnancy is a common precipitant for marriage[17]. This is reflected in the contribution of up to 10 years of pre-marital sexual exposure in the group studied, carrying a year-on-year risk of HIV which was as high as exposure of women in vulnerable inter- or post- marriage periods of their lives. Pre-marital sex for teenage girls may occur with age-mates (eg school friends) or with older men. In the teenage women sampled here (who may or may not have been married by the time of presentation at ANC), 40% of pregnancies were reported to be by a man from an age cohort at least 5 years above hers. The corresponding figure is 29% for women in their early twenties.
Qualitative work in this population suggests that women of 16 years or under have pre-marital sex only on isolated occasions: unexpected and irregular (and consequently, unprotected) [17]. Women in their twenties are more likely to have had regular boyfriends prior to marriage.
Although there appears to have been a decline in HIV prevalence between the first three and last three years of this study, the significance of this overall trend cannot be inferred directly as ANC surveillance was not continuous in all participating clinics. Areas of residence differ in their HIV infection prevalences, and some were not represented in the latter years.
The association of HIV with low parity for age may be causal in either direction: long-standing HIV infection is a biological cause of infertility and a social cause of infertility (due to widowhood), and other sexually transmitted infections increase the risk of both infertility and HIV acquisition. Primary or secondary infertility can destabilise marriage [14] and lead to periods of risky exposure. The association of HIV with low parity in this young age group was not significant, when adjusted for other factors.
We have shown that it is possible to collect useful data on sexual behaviour in an ANC setting. Questions on age at first sex may answered less well in older than in younger women, but it is the age at sexual debut in the youngest groups which give cogent information on trends, and the questions are generally well-answered in this group. The same data collection tools, with the addition of dates of termination of marriages to avoid the need for imputation, can be used in PMTCT settings to investigate whether women who choose to attend one facility in preference to another have different behavioural characteristics, which may explain any observed HIV prevalence differences by facility type. The Malawi National AIDS Commission used a variant of marital status questions developed at KPS in their last round of ANC surveillance, and it is to be hoped that other ANC surveillance programmes will follow suit, with appropriate quality controls in place.
Acknowledgements
This paper is dedicated to the late Masiya Kondowe. The Karonga Prevention Study is supported by the Wellcome Trust and LEPRA. JRG was supported by the UK Department of Health. We thank the National Health Sciences Research Committee of Malawi and the National AIDS Commission of Malawi for their continued support.
References
- 1.Asamoah-Odei E, Garcia Calleja JM, Boerma JT. HIV prevalence and trends in sub-Saharan Africa: no decline and large subregional differences. Lancet. 2004;364(9428):35–40. doi: 10.1016/S0140-6736(04)16587-2. [DOI] [PubMed] [Google Scholar]
- 2.AIDS Epidemic Update, December 2007 . AIDS Epidemic Updates. UNAIDS and WHO; 2007. p. 11. [Google Scholar]
- 3.UNAIDS/WHO Working Group of Global HIV/AIDS and STI Surveillance . Guidelines for second generation HIV surveillance: the next decade. Geneva: UNAIDS; 2000. UNAIDS/WHO Working Group of Global HIV/AIDS and STI Surveillance. [Google Scholar]
- 4.Zaba B, et al. The role of behavioral data in HIV surveillance. Aids. 2005;19(Suppl 2):S39–52. doi: 10.1097/01.aids.0000172876.74886.86. [DOI] [PubMed] [Google Scholar]
- 5.Garnett GP, et al. Behavioural data as an adjunct to HIV surveillance data. Sex Transm Infect. 2006;82(Suppl 1):i57–62. doi: 10.1136/sti.2005.016543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz T, et al. New strategies for HIV surveillance in resource-constrained settings: an overview. Aids. 2005;19(Suppl 2):S1–8. doi: 10.1097/01.aids.0000172871.80723.3e. [DOI] [PubMed] [Google Scholar]
- 7.Centres for Disease Control and Prevention (CDC) et al. New strategies for HIV/AIDS surveillance in resource-constrained countries. Addis Ababa; Ethiopia: 2004. Linking behavioural and HIV surveillance. [Google Scholar]
- 8.Glynn JR, et al. The development of the HIV epidemic in Karonga District, Malawi. Aids. 2001;15(15):2025–9. doi: 10.1097/00002030-200110190-00016. [DOI] [PubMed] [Google Scholar]
- 9.Crampin AC, et al. Trends and measurement of HIV prevalence in northern Malawi. Aids. 2003;17(12):1817–25. doi: 10.1097/00002030-200308150-00011. [DOI] [PubMed] [Google Scholar]
- 10.Jahn A, et al. Evaluation of a village-informant driven demographic surveillance system in Karonga, Northern Malawi. Demographic Research. 2007;16:2119–248. [Google Scholar]
- 11.Auvert B, et al. Ecological and individual level analysis of risk factors for HIV infection in four urban populations in sub-Saharan Africa with different levels of HIV infection. Aids. 2001;15(Suppl 4):S15–30. doi: 10.1097/00002030-200108004-00003. [DOI] [PubMed] [Google Scholar]
- 12.Glynn JR, et al. HIV risk in relation to marriage in areas with high prevalence of HIV infection. J Acquir Immune Defic Syndr. 2003;33(4):526–35. doi: 10.1097/00126334-200308010-00015. [DOI] [PubMed] [Google Scholar]
- 13.Clark S. Early marriage and HIV risks in sub-Saharan Africa. Stud Fam Plann. 2004;35(3):149–60. doi: 10.1111/j.1728-4465.2004.00019.x. [DOI] [PubMed] [Google Scholar]
- 14.Nilses C, et al. A community based study of HIV in women in rural Gutu District, Zimbabwe 1992 to 1993. Cent Afr J Med. 2000;46(2):32–7. doi: 10.4314/cajm.v46i2.8520. [DOI] [PubMed] [Google Scholar]
- 15.Jahn A, et al. HIV prevalence and risks among women who do not attend antenatal clinic. National HIV/AIDS Research Dissemination Conference; Lilongwe; Malawi. 2004. [Google Scholar]
- 16.Hemmings JM, et al. Infertility and women's life courses in northern Malawi. International Union for the Scientific Study of Population Conference; Tours; France. 2005. [Google Scholar]
- 17.Hemmings JM. London School of Hygiene and Tropical Medicine. University of London; 2007. Infertility and Women's Life Courses in Northern Malawi. PhD Thesis. [Google Scholar]