Abstract
Purpose
Although much is now understood about the molecular structure of tight junctions (TJs), little is known about the regulation of their function during neural inflammatory disease processes in vivo. The mechanisms by which leukocytes transmigrate the blood-retina barrier (BRB) without affecting endothelial permeability are controversial.
Methods
Confocal immunofluorescence microscopy of ex vivo retinal wholemounts was used to study BRB integrity during leukocyte adhesion and migration during experimental autoimmune uveoretinitis (EAU). Western blot analysis was used to measure levels of TJ proteins in EAU retina and RPE and in normal retina or RPE cultured with cytokines or chemokines.
Results
No evidence for discontinuity or other weakness of the endothelial or epithelial barrier at tricellular corners was observed, and maximum disruption of TJ protein expression was focused in retinal venules correlating with sites of leukocyte extravasation. Areas of maximum TJ protein loss in vivo also correlated with redistribution or loss of ensheathing astrocyte processes on venules but not adjacent capillaries or arterioles. Exposure of normal choroidal and retinal explants ex vivo to cytokines and chemokines alone did not downregulate total occludin-1 or claudin-3 TJ protein expression.
Conclusions
The data presented herein support an active role for leukocytes in TJ disruption and blood-retina barrier (BRB) breakdown during retinal inflammation and further implicate venule microenvironment as a key factor in leukocyte recruitment to retinal tissue in vivo.
The main function of the blood-retina barrier (BRB) is to regulate the entry of blood-borne molecules and leukocytes into the retina and to preserve ionic and immunologic homeostasis within the retinal microenvironment. Breakdown of the BRB with leukocyte recruitment is a phenomenon common to a variety of retinal diseases, and damage of the BRB may exacerbate retinal injury through formation of edema and leukocyte degranulation. Previously, we have shown that the ability of circulating, activated T cells to induce changes in the endothelium of retinal venules results in transient breakdown of the barrier and is central to immune surveillance of the central nervous system, with T-cell diapedesis occurring in the absence of cell rolling and without any reduction in shear stress.1 In experimental autoimmune uveoretinitis (EAU), cell-rolling occurs, and breakdown of BRB and infiltration of leukocytes occur in retinal venules but not arterioles.2 How and why there is leukocyte extravasation in these venules has not been not fully explained.
Occludin-1, claudins, and ZO-1 are present in tight junctions (TJs) of the blood-brain barrier (BBB),3 and have all been detected in TJs of retinal vascular endothelial cells4,5 as well as retinal pigment epithelial (RPE) cells.5-8 The mechanisms governing TJ regulation during neural inflammatory and degenerative disease processes are unclear. The role of astrocytes and their precursors in establishing and maintaining barrier function in endothelium has been demonstrated in vitro,9,10 but the connection between these cells, TJ structure, protein expression, and physiological parameters, such as shear stress during transendothelial migration of inflammatory cells in vivo, has not been studied. Even the route taken by leukocytes during diapedesis is controversial. Transmigrating leukocytes are variously reported to (1) use transcytosis and migrate through the cell body11,12; (2) to squeeze through interendothelial junctions under the control, or not, of junctional adhesion molecules (JAM-1, -2, and -3); and (3) to use points of weakness in the TJs such as the discontinuities between adjacent cells at tricellular corners.13,14 To elucidate the mechanisms involved and understand the regulation of these proteins by inflammatory cells and mediators in vivo, this study was designed to investigate the molecular and structural changes of BRB TJ proteins at various stages during chronic and acute retinal inflammation by using ex vivo tissue from normal and EAU mice.
Methods
Animals and Induction of EAU
Two mouse models of EAU were used in the present study: the acute model in B10.RIII mice and the chronic model in C57BL/6 mice. This allowed comparison of tissue from different mouse strains and mild disease with acute disease. In the acute model, mice were immunized with 50 μg of peptide 161-180 interphotoreceptor retinoid-binding protein (IRBP, SGIPYIISYLHPGNTILHVD; purity >85%; Sigma-Aldrich, Cambridge, UK) emulsified with 50 μL Freund’s complete adjuvant (CFA, H37Ra; Difco Laboratories, Detroit, MI), and retinal inflammation occurred at postimmunization (pi) days 10 to 11 and peaked at pi days 15 to 16.2,15 In the chronic model, mice were immunized with 500 μg of IRBP peptide 1-20 (GPTHLFQPSLVLDMAKVLLD; Sigma-Aldrich) emulsified in complete Freund’s adjuvant subcutaneously. Mice were administered an additional intraperitoneal injection of 100 μL (1.5 μg) of Bordetella pertussis toxin. In that model, retinal inflammation occurred at days 16 to 18 pi and peaked at days 21 to 28 pi.16 All animals, weighing approximately 20 g, 8 to 12 weeks old, were supplied by the Medical Research Facility of Aberdeen University and maintained in accordance with Home Office Regulations for Animal Experimentation (UK) and with the ARVO Statement for the Use of Animal in Ophthalmic and Vision Research.
Immunohistochemistry of Retinal Wholemounts for Confocal Fluorescence Laser Scanning Microscopy
The animals were killed by CO2 inhalation and the eyes enucleated. Retinas and choroid (including the RPE) were dissected and processed according to the method of Morcos et al.5 In brief, the cornea, crystalline lens, and vitreous were removed in ice-cold phosphate-buffered saline (PBS), and the eyecups were transferred to fresh, ice-cold PBS. The retina and choroid were dissected and fixed in 100% ethanol for 30 minutes at 4°C. The tissues were then delipidated for 5 minutes in 100% ice-cold acetone.
After they were washed for 30 minutes at room temperature in PBS, samples were blocked and permeabilized with 5% (wt/vol) bovine serum albumin (BSA) with 0.3% (vol/vol) Triton in PBS at room temperature for 1 to 2 hours. Samples were then incubated with mouse anti-GFAP cocktail (1:50; BD-Bioscience, Oxford, UK) and/or rabbit polyclonal antibodies to occludin-1 (cat. no. 71-1500; Zymed, South San Francisco, CA), claudin-1/3 (cat. no. 71-7800; Zymed), and ZO-1 (cat. no. 61-7300; Zymed) that had been diluted 1:100 with PBS containing 1% BSA. In the case of glial fibrillary acidic protein (GFAP) staining, samples were incubated at 4°C for 3 days. For all other staining, samples were incubated at 4°C overnight with first antibodies. The tissues were washed for 30 minutes at room temperature and incubated for 1 hour with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin (Ig; 1:50 dilution in 1% BSA-PBS) and RPE rat anti-mouse Ig (1:100; BD-Bioscience). After another 30-minute wash in PBS, the tissues were mounted in antifade medium (Vectashield; Vector Laboratories, Burlingame, CA). Samples were then examined with a confocal microscope (LSM 510 META; Carl Zeiss Meditec, Göttingen, Germany). To image the whole structure of TJ proteins in a particular vessel segment. Z-stacks were taken during image acquisition. As a control, tissue was incubated with rabbit Ig or in the absence of primary antibodies. No immunoreactivity was detected in the control samples.
To study the relationship between leukocyte adhesion, transmigration, and TJ protein expression, animals were gently perfused with PBS before removal of the eyes. Retinal wholemounts, prepared as just described, were then incubated with FITC anti-occludin-1 (1:100; Zymed) and PE anti mouse CD44 (1:100; BD Bioscience), in 1% BSA-PBS in the dark at 4°C overnight. After they were washed, samples were mounted and observed as described earlier.
Western Blot Analysis
Retinas and choroid/RPE cell layers were carefully dissected from normal or EAU mouse eyes, and whole cell lysates were prepared for Western blot analysis. To determine effects of cytokines or chemokines alone, retinas and choroids/RPE from normal mouse eyes were cultured for 24 hours with optimized concentrations of cytokines (TNF-α, 100 ng/mL17; IL-β, 100 ng/mL; IFN-γ, 100 ng/mL18; or chemokines (RANTES [regulated on activation normal T-cell expressed and secreted], 10 ng/mL; MCP-1, 10 ng/mL; and MIP-1α, 10 ng/mL). Lysates of retinal tissues were prepared with 0.1% Triton X-100 extraction buffer18 containing phenylmethylsulfonyl fluoride (PMSF) and dithiothreitol (DTT). Protein concentration in tissue lysates was determined by using the Bradford assay (Bio-Rad, Hercules, CA), and the protein concentrations were adjusted to allow equal total protein loading on the gels. Samples were mixed with an equal volume of sodium dodecyl sulfate (SDS) sample buffer and analyzed for TJ protein expression by SDS-polyacrylamide gel electrophoresis. Proteins were transferred electrophoretically to ECL membranes (Bio-Red) and TJ proteins (claudin-1/3, occludin-1, and ZO-1) identified with rabbit polyclonal antibodies from Zymed Laboratories at 1:3000 in 6% (wt/vol) BSA-TBS. GFAP was detected with mouse anti GFAP clone 4A11 (BD Biosciences). Chemiluminescent membranes were washed and incubated with horseradish-peroxidase (HRP)-tagged goat-anti-rabbit Ig or HRP tagged goat anti-mouse Ig and visualized (ECL; Amersham Biosciences, Piscataway, NJ). To distinguish between claudin-1 and -3 additional experiments were performed with a specific anti-claudin-1 antibody (cat. no. RB-1675; Stratech Scientific, Ltd., Cambridge, UK) and an anti-claudin-3 antibody (cat. no. 34-1700; Zymed) that became available during the study.
Image Analysis and Statistics
Confocal images were analyzed on computer (Image Pro Plus system; Media Cybernetics, Silver Spring, MD). Western blot images were acquired (GeneSnap system; SynGene, Cambridge, UK), and densitommetry was performed (Gene Tools software; SynGene). For quantitative data, each assay was repeated at least three times. Data are presented as the mean ± SEM. Probabilities between control and cytokine- or chemokine-treated groups were calculated with the Dunnett multiple comparison test. P < 0.05 was considered statistically significant.
Results
TJ Protein Expression in Retinal Venules and Retinal Arterioles during EAU
In vivo expression of TJ proteins claudin-1/3, occludin-1, and ZO-1 was evaluated by immunohistochemistry in the tissues of retina and choroid (including RPE) separately. In the B10.RIII and C57BL/6 nonimmunized control mice, all retinal vessels (arterioles, venules, and capillaries) were positive for TJ proteins claudin-1/3 (Fig. 1A), occludin-1 (Figs. 1C, 1G), and ZO-1 (Fig. 1E). TJ proteins were evenly distributed along the endothelial cell junction areas with no obvious areas of weakness. In fact, staining appeared to be even stronger in tricellular corners (Figs. 1G, 1H). These TJ proteins were disrupted only in retinal venules during both chronic and acute EAU (Figs. 1B, 1D, 1F). This corresponds with our earlier observations that adhesion molecule upregulation in EAU occurs largely at post-capillary venules.2 Only minimal changes were observed in the capillaries; retinal arterioles appeared unaffected (Fig. 2). These observations also correlate with sites of BRB breakdown and leukocyte infiltration.2 Maximum disruption of TJ proteins occurred at peak disease in both B10.RIII mice and C57BL/6 mice (data not shown).
Figure 1.
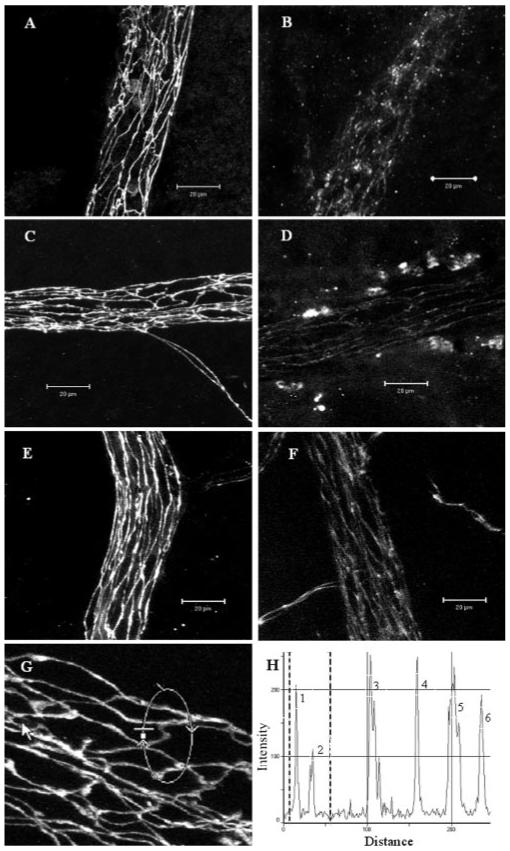
Confocal images of TJ protein expression in retinal venules of normal and EAU mice. Retinal wholemounts from normal nonimmunized B10R.III mice (A, C, E, G) or day 12 pi mice (B, D, F) were stained with anti claudin-1/3 (A, B), anti occludin-1 (C, D, G), and anti ZO-1 (E, F). All TJ proteins were evident at the interfaces between adjacent endothelial cells of retinal vessels from normal B10R.III mice and only weakly appeared in inflamed venules. (H) Histogram of fluorescence intensity of occludin-1 along the circular track shown in (G). Peaks 1 and 2 are two junction sites within the marked area in (G). Peaks 3, 4, and 6 are three tricellular corner sites. Fluorescence intensity of occludin-1 was not reduced in the tricellular areas. Images shown are reconstructions of a series of Z-stacks (15-30-μm thickness) of retinal venule segments. Bars, 20 μm.
Figure 2.
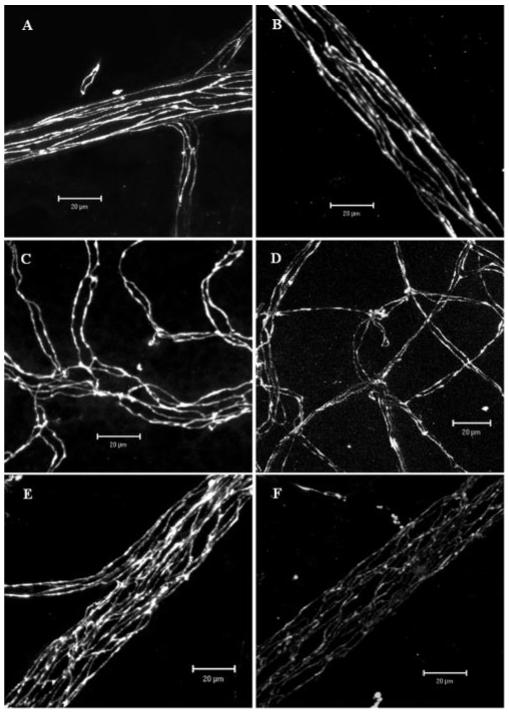
Confocal images of TJ protein occludin-1 in retinal vessels of normal and EAU mice. (A, B) arterioles; (C, D) capillaries; (E, F) venules. (A, C, E) Normal control; (B, D, F) EAU day 12 pi. Note the remarkable reduction of occludin-1 expression in retinal venules of EAU mouse. Images shown are reconstructions of a series of Z-stacks (15-30 μm thickness). Bars, 20 μm.
Western blot analysis of retinas confirmed that the TJ disruption observed by immunofluorescence correlated with reduced claudin-1/3 and occludin-1 protein expression (Fig. 3), suggesting downregulation of these TJ proteins during EAU. In contrast, although ZO-1 stained weakly in the venules of EAU retina, Western blot data showed no significant overall reduction in ZO-1 protein expression. This is consistent with diffuse redistribution of the protein within the endothelial cells, or changes in expression not significant enough to be detected by Western blot analysis.
Figure 3.
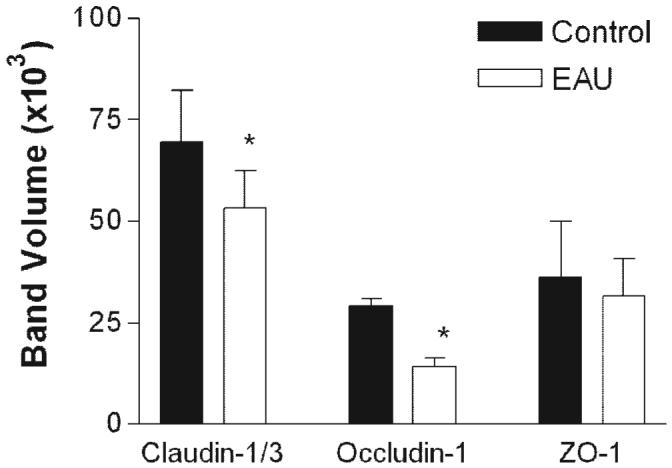
Western blot analysis for TJ proteins in retinas from normal and EAU mice. Retinas were dissected from the choroids-RPE tissue in normal or EAU mice (C57BL/6 milder disease) and proteins extracted for Western blot analysis with claudin-1/3, occludin-1, and ZO-1. Data shown are expressed as the mean ± SEM. n = 3 mice, *P < 0.05 Student’s t-test.
TJ Proteins in RPE Cells during EAU
Consistent with previous observations in rabbit eyes,5 mouse RPE cells were also found to express claudin-1/3 (Fig. 4A), occludin-1 (Fig. 4C), and ZO-1 (Fig. 4E). The RPE forms a monolayer of cells in vivo. The cells are typically pentagonal or hexagonal in shape, and TJ proteins appeared evenly expressed around their entire circumference (Figs. 4A, 4C, 4E) with no evidence of weakness in the tricellular corners (Figs. 4G, 4H). Protein expression within these cells was significantly disordered during EAU, with loss of protein from cell borders along one or more sides of the cells (Figs. 4B, 4D, 4F). However, Western blot analysis of choroid/RPE tissue from EAU eyes showed that in contrast to retinal vessels, there was no significant reduction in overall claudin-1/3, occludin-1, or ZO-1 proteins, indicating that, although TJs had broken down, proteins were redistributed rather than downregulated in RPE during intraocular inflammation (Fig. 5).
Figure 4.
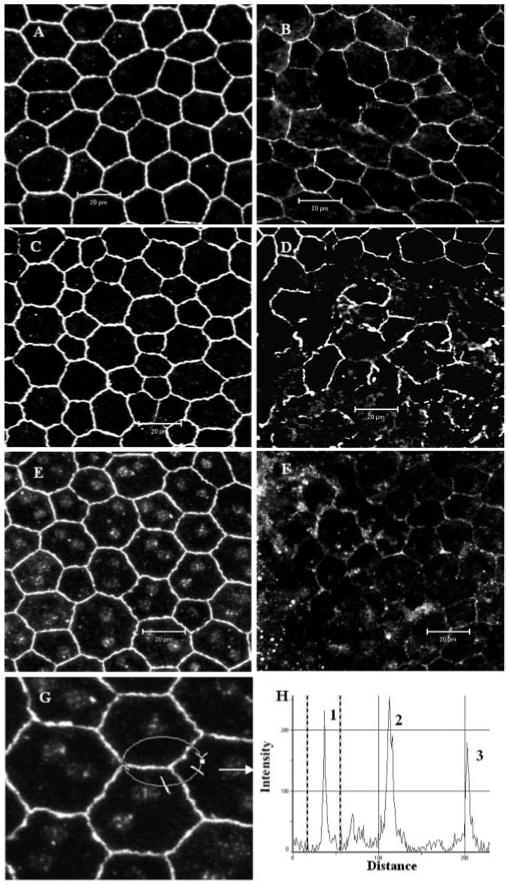
Confocal images of TJ protein expression in RPE cells of normal and EAU mice. Wholemounts of choroids (include RPE) from normal nonimmunized B10R.III mice (A, C, E, G) or day 12 pi mice (B, D, F) were stained with anti claudin-1/3 (A, B), anti occludin-1 (C, D), and anti ZO-1 (E, F, G). All TJ proteins were detected around the entire circumference of RPE cells from normal B10R.III mice and markedly disrupted in inflamed RPE cells. (H) Histogram of fluorescence intensity of ZO-1 along the circular track shown in (G). Peaks 1 and 3 are two cell border sites and peak 2 is the tricellular corner site. Images are reconstructions of a series of Z-stacks (12-μm thickness). Bars, 20 μm.
Figure 5.
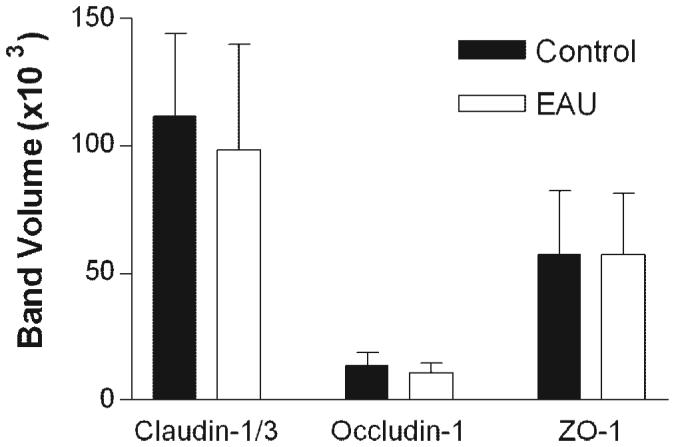
Quantification of results in a Western blot analysis of TJ proteins in RPE cells from normal and EAU mice. RPE-choroid tissues were dissected from normal or EAU mice (C57BL/6 milder disease) and proteins extracted for Western blot analysis with claudin-1/3, occludin-1, and ZO-1. Data are expressed as the mean ± SEM; n = 3 mice.
Effect of Cytokines and Chemokines on Retinal TJ Proteins Ex Vivo
Cytokines (TNF-α, IFN-γ, and IL-1β)19-21 and chemokines (MIP-1α, MCP-1, and RANTES)22,23 play critical roles during the pathogenesis of EAU. Although some growth factors and oxidative stress are reported to affect RPE TJs.24,25 Whether cytokines and chemokines can directly affect TJ protein expression during EAU is unknown. Retinas and choroids (including RPE) from normal B10.RIII and C57BL/6 mice were cultured in vitro with various cytokines (TNF-α, IFN-γ, and IL-1β) and chemokines (MIP-1α, MCP-1, and RANTES) for 24 hours, and expression of TJ proteins was evaluated by Western blot analysis. With rabbit anti-claudin-1/3, two bands were detected in both retinal and RPE samples in Western blot analysis. In the retinal tissue, the top band was stronger than the bottom band, whereas in the RPE tissue, the bottom band was stronger then the top band (Fig. 6). Further comparisons conducted with the more specific anti-claudin-1 antibody and anti-claudin-3 antibodies showed that the top band was claudin-3 (22 kDa) and the bottom band was claudin-1 (20 kDa; data not shown), suggesting that, in the retina, claudin-3 is predominant, whereas in RPE cells, claudin-1 is predominant, consistent with differential distribution of claudin proteins in separate tissues. Table 1 shows that retinal endothelial cell TJ proteins were not downregulated by any of the cytokines and chemokines tested. In contrast, in RPE cells, both IL-1β and MCP-1 significantly reduced ZO-1 (P < 0.02) protein expression. However, TJ regulatory proteins occludin-1 and claudin-1/3 expression remained unaffected by cytokine and chemokine treatment.
Figure 6.
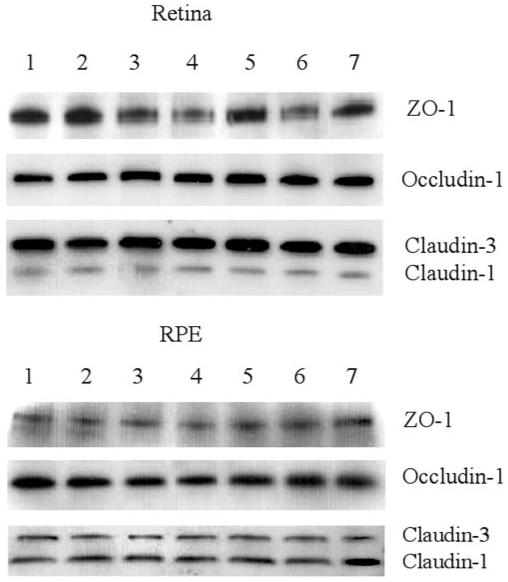
Western blot analysis of ex vivo retinas and RPE cells for TJ proteins ZO-1, occludin-1, and claudin-1/3 after cytokine or chemokine treatment. Retinal and choroid-RPE tissues were dissected from normal mouse eyes and incubated for 24 hours in the presence or absence of different cytokines or chemokines. Proteins were extracted for Western blot analysis. Row 1, control; row 2, IFN-γ; row 3, TNF-α; row 4, IL-1β; row 5, MCP-1; row 6, MIP-1α; and row 7, RANTES. Data are representative of three experiments. Bands representing either claudin-1 (20 kDa) or claudin-3 (22 kDa) were differentiated in blots, by using specific claudin-1 and claudin-3 antibodies that became available during the period of the study (data not shown).
TABLE 1.
Densitometric Data (Pixel Intensity) of Western Blot Analyses
| Control | IFN-γ | TNF-α | IL-1β | MCP-1 | MIP-1α | RANTES | |
|---|---|---|---|---|---|---|---|
| Retina | |||||||
| ZO-1 | 17.13 ± 5.65 | 13.01 ± 3.82 | 17.75 ± 5.51 | 14.75 ± 6.59 | 11.70 ± 4.85 | 22.76 ± 8.74 | 18.52 ± 6.48 |
| Occludin-1 | 55.42 ± 12.73 | 64.92 ± 11.05 | 58.10 ± 13.59 | 51.29 ± 11.57 | 53.57 ± 6.47 | 38.06 ± 8.22 | 52.21 ± 13.06 |
| Claudin-1 | 16.25 ± 5.72 | 11.33 ± 4.16 | 10.71 ± 1.72 | 11.64 ± 6.01 | 13.92 ± 4.96 | 16.48 ± 3.94 | 20.11 ± 6.53 |
| Claudin-3 | 67.17 ± 5.15 | 63.32 ± 2.89 | 68.22 ± 11.60 | 49.53 ± 6.45 | 73.18 ± 15.57 | 73.60 ± 10.63 | 70.18 ± 9.87 |
| RPE cells | |||||||
| ZO-1 | 33.39 ± 4.32 | 31.84 ± 2.76 | 28.04 ± 3.99 | 18.02 ± 2.83* | 18.57 ± 2.41* | 27.37 ± 5.60 | 26.38 ± 7.51 |
| Occludin-1 | 28.82 ± 17.93 | 24.50 ± 11.21 | 20.28 ± 10.05 | 14.37 ± 6.17 | 16.49 ± 5.85 | 19.19 ± 5.37 | 24.32 ± 8.23 |
| Claudin-1 | 50.75 ± 6.73 | 36.16 ± 17.38 | 47.84 ± 20.33 | 39.21 ± 8.59 | 58.97 ± 12.00 | 53.55 ± 6.58 | 51.10 ± 3.92 |
| Claudin-3 | 20.09 ± 9.95 | 10.16 ± 3.51 | 12.75 ± 2.85 | 18.41 ± 4.06 | 17.90 ± 9.23 | 23.13 ± 11.45 | 15.65 ± 5.95 |
P < 0.05 compared with the control group of the same protein. Dunnett multiple comparison test.
Data are expressed as the mean ± SEM (n = 3 × 103).
Effect of Leukocyte-Adhesion and Transmigration in Retinal Venules on Redistribution of TJ Protein
Leukocyte infiltration of the retina is one of the main hallmarks of EAU and other central nervous system (CNS) inflammatory conditions. It is not yet clear whether BRB breakdown precedes infiltration or is a consequence of infiltration. To further understand the regulation of TJ proteins during EAU, we asked whether in vivo direct contact of leukocyte to retinal vascular endothelial cells and transmigration of leukocytes during inflammation could induce redistribution or even loss of TJ proteins. Day 9 pi B10.RIII mice (early stage of EAU) were carefully perfused, and retinal wholemounts were double stained with CD44 and occludin-1. Direct contact by a CD44+ leukocyte induced diffusive expression in the cytoplasm and reduced expression in cell junctions of occludin-1 in retinal venule endothelial cells (Fig. 7A). Transendothelial migration of a CD44+ leukocyte caused total loss of occludin-1 from the junction area in vascular endothelial cells and diffuse expression in adjacent vascular endothelial cells (Fig. 7B). This focal loss of occludin-1 protein appeared to have been reconstituted in other sites where leukocytes had already transmigrated into the retinal parenchyma (Fig. 7C).
Figure 7.
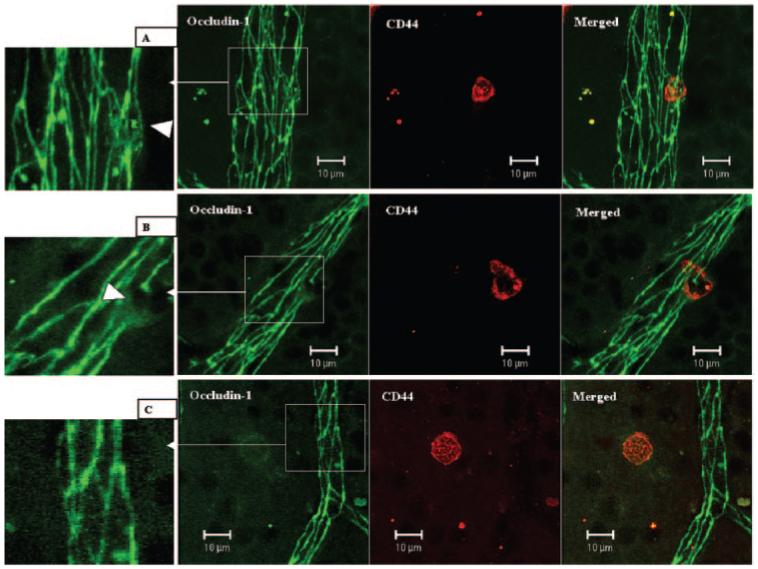
Changes of occludin-1 in retinal venules during leukocyte adhesion and transendothelial cell migration. Day 9 pi EAU mice were gently perfused with PBS. Retinal wholemounts were double stained with anti-occludin-1 (FITC) and anti-CD44 (R-PE). (A) One CD44+ leukocyte adhering to a retinal venule. TJ protein occludin-1 is diffuse in the adhering area (inset, arrowhead). (B) One CD44+ leukocyte was in the process of transendothelial cell migration. TJ protein occludin-1 was absent in the transmigration area and diffuse in the adjacent area (inset, arrowhead). (C) One CD44+ cells had migrated into the tissue, and there was no occludin-1 disruption in surrounding vessels. Images are reconstructions from a series of Z-stacks (15-40-μm thickness). Bars, 10 μm.
Redistribution of Astrocytes and Loss of Endothelial Cell/Astrocyte Foot Process Contact during EAU
Astrocytes are known to play important roles in the formation and maintenance of TJ proteins in vitro.26-28 Astrocytes in the control nonimmunized B10.RIII and C57BL/6 mouse retina were found in the ganglion and nerve fiber layers. They were evenly distributed, had a predominantly stellate morphology and sent numerous processes or end feet to contact both arterioles and venules. These end feet formed reticular structures ensheathing the retinal vessels (Fig. 8A). In inflamed retina, there was a loss of even distribution of astrocyte processes in the parenchyma and loss of even ensheathment of vessels in retinal venules (Fig. 8B). These regions of disensheathment of vessels were also colocalized with the regions of TJ protein occludin-1 downregulation (Fig. 8B). Astrocyte contact with adjacent capillaries was retained, and TJ integrity appeared to be intact, despite the presence of an extensive inflammatory infiltrate. Western blot analysis of GFAP protein expression in retinal tissue showed that overall GFAP protein expression was slightly downregulated in inflamed retina (Fig. 8C), which is in line with the reduced or altered distribution of GFAP filaments observed by immunofluorescence.
Figure 8.
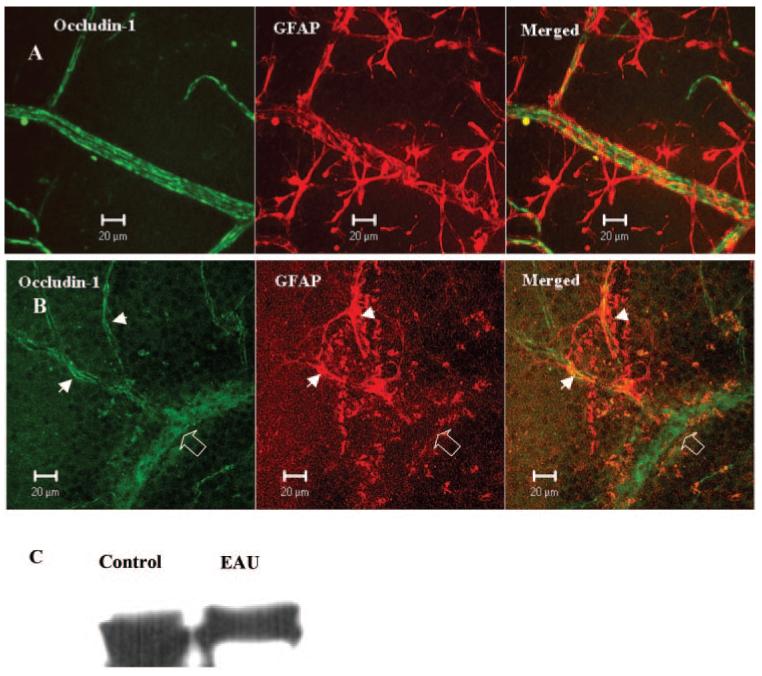
Occludin-1 and GFAP immunoreactivity in normal and EAU mouse retina. Retinal wholemounts from a normal B10R.III mouse (A) and a day 12 pi EAU mouse (B) were stained with anti-occludin-1 and anti-GFAP. Samples were observed by confocal microscopy. Note the total loss of GFAP staining in the vessel segment with occludin-1 disruption (diffuse occludin-1 staining, open arrow) and the preservation of occludin-1 in capillaries where GFAP astrocytes are maintained intact (arrowheads). Images shown are reconstructions from a series of Z-stacks (40-μm thickness). (C) Western blot of retinal tissue from control and EAU mice showing reduced GFAP protein expression in EAU retina.
Discussion
In this study, using a mouse model of retinal inflammation, BRB TJ disruption and TJ protein downregulation and redistribution associated with leukocyte extravasation in vivo differed in several respects from findings in some studies investigating TJ disruption and leukocyte transmigration in vitro. 13,29 The TJ proteins claudin-1/3, occludin-l, and ZO-1 were all highly expressed by both retinal endothelium and RPE cells, but with no evidence of discontinuity or other weakness of the endothelial or epithelial barrier at tricellular corners. Indeed, expression of TJ proteins at these points appeared to be stronger than along cell borders even in the venules where most of the leukocyte transmigration was occurring (Figs. 1, 2). Our data also indicate that proinflammatory cytokines and chemokines alone (including IFN-γ, IL-1β, MIP-1α, and MCP-1) do not downregulate claudin-1/3 or occludin-1 expression in ex vivo endothelium or epithelium.
The regulation of TJ proteins in vivo, particularly during inflammation, is not yet fully understood. It has been suggested that cytokines such as IL-1β, TNF-α, or IFN-γ play important roles, as injection of these cytokines could induce breakdown of BRB.19,30,31 Recent studies by Song and Pachter32 showed that MCP-1 downregulated ZO-1 but not occludin in both cultured brain microvascular endothelial cells and fresh isolated brain microvessels. This is in agreement with our ex vivo studies in choroid-RPE tissue (Fig. 6, Table 1). In retinal endothelial tissue however, none of the tested cytokines and chemokines showed significant effect on TJ protein expression (Table 1). Confocal analysis of retinal wholemounts showed downregulation of TJ proteins only in retinal venules but not arterioles. If only venules were affected by the treatment, then Western blot analysis may not be sensitive enough to pick it up. Whether TJ morphology in intact venules can be compromised by cytokine or chemokine treatment alone requires immunohistochemical confirmation, and these studies are under way. Within the retinal tissue, arterioles and venules are anatomically close to each other, so any soluble factors such as cytokines and chemokines that affect venules would be predicted also to have an affect on the arterioles. In other words, the endothelial cells of retinal venules and arterioles are subjected to the same condition as any soluble factors within the inflamed retina. It is possible that some chemokines are locally immobilized on the endothelial cells of inflamed venules by binding glycosaminoglycans and Duffy antigen/receptor for chemokine (DARC) docking on vascular endothelial cells.33 However, their main biological role is believed to be in leukocyte activation. There is no evidence so far to suggest that they can affect endothelial cell TJs. Alternatively, other factors within arterioles or capillary endothelium may counteract the soluble mediators present.
The difference in the expression of TJ proteins between retinal arterioles and venules during EAU indicates that factors that affect TJ protein expression are presented locally in the diseased venules. Direct contact of leukocyte to activated endothelial cells must induce signal transduction.34,35 However, whether this could trigger disruption or reorganization of TJ proteins is controversial. Other in vivo observations have shown that leukocyte infiltration is highly associated with loss of TJ proteins.36-38 In our study, we also showed significant downregulation of the TJ tetraspan integral proteins claudin-1/3 and occludin-1 in inflamed endothelium, indicating loss of intercellular adhesive function in affected cells. Perhaps unexpectedly, no significant change in expression of the scaffold protein ZO-1 was found in these tissues, suggesting that redistribution rather than downregulation of protein had occurred. Shuttling of ZO-1 between the nucleus and the plasma membrane has been suggested as a mechanism for establishing and regulating TJ during differentiation and growth. Nuclear ZO-1 is clearly present within the nuclei of normal RPE cells in explants (Fig. 4). ZO-1 expression can be downregulated by cytokines such as MCP-1 and IL-1β,32 but it can also be up regulated by cytokines such as IL-15.39 RPE cells produce IL-15 in response to inflammatory stimuli40 and would also be present within the inflammatory infiltrates in the choroid and retina of our EAU mice, providing an explanation for maintenance of ZO-1 protein levels within ex vivo tissues. Why claudin-1/3 and occludin-1 were not downregulated in the EAU choroid/RPE as occurred in EAU retina is less clear, but it may relate to the absence of astrocyte factors in the outer retina.
In the present study, in a murine retinal inflammation model, direct contact of leukocytes with retinal venule endothelial cells induced diffusive expression of the TJ protein occludin-1 (Fig. 7). As the leukocyte migrates into the retinal parenchyma, occludin-1 proteins reassemble to reseal the barrier.41 It is possible that the apparent loss of staining in confocal sections could represent distortion of the intact junction out of the plane of focus; however, reconstruction of confocal Z-stacks reveal that staining is lost from all planes (Fig. 7). These observations would suggest that TJs are indeed broken down by leukocytes in vivo but can reassemble rapidly after leukocyte diapedesis. This observation is in accordance with real-time imaging experiments by Shaw et al.42 conducted in vitro, but under conditions of flow. These experiments showed that junctions open and reseal within 5 minutes to allow monocyte transmigration. Shaw’s model also showed that monocytes did not preferentially migrate at corners, and that cell contact created a gap in the TJ to allow transmigration. Shear stress has been implicated in TJ formation and may account for the discrepancies in TJ protein distribution in studies in vitro.
During the peak stage of retinal inflammation, however, the TJ proteins claudin-1/3 and occludin-1 were significantly downregulated in all retinal venules. This could not be explained by the leukocyte-endothelial cell interaction theory presented herein, which seems to be focal and transient. Astrocytes have been suggested to contribute greatly to the development and maintenance of the BBB.26,28 In the inflamed retina in this study, astrocytes lost their even distribution in retinal parenchyma. Many venules lost their astrocyte contacts, and these changes colocalized with the loss of TJ protein occludin-1. These observations suggest that during retinal inflammation redistribution of astrocytes and disensheathment of retinal venules greatly affects maintenance of TJ proteins in retinal venules. However, what causes redistribution and disensheathment of retinal astrocytes is currently unknown and should be investigated further, particularly as the effect appears to be localized mainly to venules and areas of active leukocyte infiltration.
All our data, in this study and our previous studies of leukocyte trafficking, are consistent with a major role for venule endothelium in leukocyte recruitment to the retina. Venules have also been identified as the major site for inflammatory cell extravasation in other models and diseases. The effect is probably localized within the luminal surface of the venules, as circulating mediators in the blood would presumably also affect arterioles and capillaries. The effect is also apparently closely regulated within the retinal parenchyma, as soluble mediators produced within the lesions do not apparently affect the abluminal aspects of the arterioles and capillaries. Previously, we have shown that in retinal venules ICAM-1 was upregulated 48 hours before clinical disease, which was followed by adhesion of leukocytes and breakdown of BRB.2 Chemokines have also been implicated, and their distribution must also play a pivotal role in leukocyte recruitment. Leukocyte rolling and infiltration were also observed in retinal venules but not in arterioles.43 Those observations suggest that retinal venules but not arterioles actively participate in the recruitment of leukocytes to the retinal parenchyma.
In conclusion, the data we present support an active role for leukocytes in TJ disruption and BRB breakdown during retinal inflammation and further implicate the venule microenvironment as a key factor in leukocyte recruitment to retinal tissue in vivo.
Acknowledgments
The authors thank Carol Wallace and Elizabeth Muckersie for technical assistance.
Supported by The Wellcome Trust Grant 068109.
Footnotes
Disclosure: H. Xu, None; R. Dawson, None; I.J. Crane, None; J. Liversidge, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
References
- 1.Xu H, Manivannan A, Liversidge J, Sharp PF, Forrester JV, Crane IJ. Requirements for passage of T lymphocytes across non-inflamed retinal microvessels. J Neuroimmunol. 2003;142:47–57. doi: 10.1016/s0165-5728(03)00258-3. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, Forrester JV, Liversidge J, Crane IJ. Leukocyte trafficking in experimental autoimmune uveitis: breakdown of blood-retinal barrier and upregulation of cellular adhesion molecules. Invest Ophthalmol Vis Sci. 2003;44:226–234. doi: 10.1167/iovs.01-1202. [DOI] [PubMed] [Google Scholar]
- 3.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vasc Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 4.Barber AJ, Antonetti DA, Gardner TW, the Penn State Retina Research Group Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Invest Ophthalmol Vis Sci. 2000;41:3561–3568. [PubMed] [Google Scholar]
- 5.Morcos Y, Hosie MJ, Bauer HC, Chan-Ling T. Immunolocalization of occludin and claudin-1 to tight junctions in intact CNS vessels of mammalian retina. J Neurocytol. 2001;30:107–123. doi: 10.1023/a:1011982906125. [DOI] [PubMed] [Google Scholar]
- 6.Tserentsoodol N, Shin BC, Suzuki T, Takata K. Colocalization of tight junction proteins, occludin and ZO-1, and glucose transporter GLUT1 in cells of the blood-ocular barrier in the mouse eye. Histochem Cell Biol. 1998;110:543–551. doi: 10.1007/s004180050316. [DOI] [PubMed] [Google Scholar]
- 7.Williams CD, Rizzolo LJ. Remodeling of junctional complexes during the development of the outer blood-retinal barrier. Anat Rec. 1997;249:380–388. doi: 10.1002/(SICI)1097-0185(199711)249:3<380::AID-AR9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 8.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 9.Wolburg H, Neuhaus J, Kniesel U, et al. Modulation of tight junction structure in blood-brain barrier endothelial cells: effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci. 1994;107:1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- 10.Hamm S, Dehouck B, Kraus J, et al. Astrocyte mediated modulation of blood-brain barrier permeability does not correlate with a loss of tight junction proteins from the cellular contacts. Cell Tissue Res. 2004;315:157–166. doi: 10.1007/s00441-003-0825-y. [DOI] [PubMed] [Google Scholar]
- 11.Greenwood J, Howes R, Lightman S. The blood-retinal barrier in experimental autoimmune uveoretinitis: leukocyte interactions and functional damage. Lab Invest. 1994;70:39–52. [PubMed] [Google Scholar]
- 12.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson-Leger C, Aurrand-Lions M, Imhof BA. The parting of the endothelium: miracle, or simply a junctional affair? J Cell Sci. 2000;113:921–933. doi: 10.1242/jcs.113.6.921. [DOI] [PubMed] [Google Scholar]
- 14.Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. Leukocyte transendothelial migration: a junctional affair. Semin Immunol. 2002;14:105–113. doi: 10.1006/smim.2001.0347. [DOI] [PubMed] [Google Scholar]
- 15.Silver PB, Chan CC, Wiggert B, Caspi RR. The requirement for pertussis to induce EAU is strain-dependent: B10.RIII, but not B10: a mice, develop EAU and Th1 responses to IRBP without pertussis treatment. Invest Ophthalmol Vis Sci. 1999;40:2898–2905. [PubMed] [Google Scholar]
- 16.Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol. 2002;161:1669–1677. doi: 10.1016/S0002-9440(10)64444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wachtel M, Bolliger MF, Ishihara H, Frei K, Bluethmann H, Gloor SM. Down-regulation of occludin expression in astrocytes by tumour necrosis factor (TNF) is mediated via TNF type-1 receptor and nuclear factor-kappaB activation. J Neurochem. 2001;78:155–162. doi: 10.1046/j.1471-4159.2001.00399.x. [DOI] [PubMed] [Google Scholar]
- 18.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luna JD, Chan CC, Derevjanik NL, et al. Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: comparison with vascular endothelial growth factor, tumor necrosis factor alpha, and interleukin-1beta-mediated breakdown. J Neurosci Res. 1997;49:268–280. doi: 10.1002/(sici)1097-4547(19970801)49:3<268::aid-jnr2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Caspi RR, Sun B, Agarwal RK, et al. T cell mechanisms in experimental autoimmune uveoretinitis: susceptibility is a function of the cytokine response profile. Eye. 1997;11:209–212. doi: 10.1038/eye.1997.53. [DOI] [PubMed] [Google Scholar]
- 21.Dick AD, Duncan L, Hale G, Waldmann H, Isaacs J. Neutralizing TNF-alpha activity modulates T-cell phenotype and function in experimental autoimmune uveoretinitis. J Autoimmun. 1998;11:255–264. doi: 10.1006/jaut.1998.0197. [DOI] [PubMed] [Google Scholar]
- 22.Crane IJ, McKillop-Smith S, Wallace CA, Lamont GR, Forrester JV. Expression of the chemokines MIP-1alpha, MCP-1, and RANTES in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2001;42:1547–1552. [PubMed] [Google Scholar]
- 23.Keino H, Takeuchi M, Kezuka T, Yamakawa N, Tsukahara R, Usui M. Chemokine and chemokine receptor expression during experimental autoimmune uveoretinitis in mice. Graefes Arch Clin Exp Ophthalmol. 2003;241:111–115. doi: 10.1007/s00417-002-0556-x. [DOI] [PubMed] [Google Scholar]
- 24.Jin M, Barron E, He S, Ryan SJ, Hinton DR. Regulation of RPE intercellular junction integrity and function by hepatocyte growth factor. Invest Ophthalmol Vis Sci. 2002;43:2782–2790. [PubMed] [Google Scholar]
- 25.Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:675–684. doi: 10.1167/iovs.03-0351. [DOI] [PubMed] [Google Scholar]
- 26.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 27.Tao-Cheng JH, Nagy Z, Brightman MW. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci. 1987;7:3293–3299. doi: 10.1523/JNEUROSCI.07-10-03293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin LL, Hall DE, Porter S, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 30.Bamforth SD, Lightman S, Greenwood J. The effect of TNF-alpha and IL-6 on the permeability of the rat blood-retinal barrier in vivo. Acta Neuropathol (Berl) 1996;91:624–632. doi: 10.1007/s004010050476. [DOI] [PubMed] [Google Scholar]
- 31.Bamforth SD, Lightman SL, Greenwood J. Interleukin-1 beta-induced disruption of the retinal vascular barrier of the central nervous system is mediated through leukocyte recruitment and histamine. Am J Pathol. 1997;150:329–340. [PMC free article] [PubMed] [Google Scholar]
- 32.Song L, Pachter JS. Monocyte chemoattractant protein-1 alters expression of tight junction-associated proteins in brain microvascular endothelial cells. Microvasc Res. 2004;67:78–89. doi: 10.1016/j.mvr.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 34.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 35.Greenwood J, Etienne-Manneville S, Adamson P, Couraud PO. Lymphocyte migration into the central nervous system: implication of ICAM-1 signalling at the blood-brain barrier. Vasc Pharmacol. 2002;38:315–322. doi: 10.1016/s1537-1891(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 36.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–1257. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 37.Boven LA, Middel J, Verhoef J, De Groot CJ, Nottet HS. Monocyte infiltration is highly associated with loss of the tight junction protein zonula occludens in HIV-1-associated dementia. Neuropathol Appl Neurobiol. 2000;26:356–360. doi: 10.1046/j.1365-2990.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- 38.Plumb J, McQuaid S, Mirakhur M, Kirk J. Abnormal endothelial tight junctions in active lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol. 2002;12:154–169. doi: 10.1111/j.1750-3639.2002.tb00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishiyama R, Sakaguchi T, Kinugasa T, et al. Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions. J Biol Chem. 2001;276:35571–35580. doi: 10.1074/jbc.M106013200. [DOI] [PubMed] [Google Scholar]
- 40.Kumaki N, Anderson DM, Cosman D, Kumaki S. Expression of interleukin-15 and its receptor by human fetal retinal pigment epithelial cells. Curr Eye Res. 1996;15:876–882. doi: 10.3109/02713689609017629. [DOI] [PubMed] [Google Scholar]
- 41.Lacaz-Vieira F, Jaeger MM, Farshori P, Kachar B. Small synthetic peptides homologous to segments of the first external loop of occludin impair tight junction resealing. J Membr Biol. 1999;168:289–297. doi: 10.1007/s002329900518. [DOI] [PubMed] [Google Scholar]
- 42.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 43.Xu H, Manivannan A, Goatman KA, et al. Reduction in shear stress, activation of the endothelium, and leukocyte priming are all required for leukocyte passage across the blood-retina barrier. J Leukoc Biol. 2004;75:224–232. doi: 10.1189/jlb.1002479. [DOI] [PubMed] [Google Scholar]


