Abstract
Masked priming is used in psycholinguistic studies to assess questions about lexical access and representation. We present two masked priming experiments using MEG. If the MEG signal elicited by words reflects specific aspects of lexical retrieval, then one expects to identify specific neural correlates of retrieval that are sensitive to priming. To date, the electrophysiological evidence has been equivocal. We report findings from two experiments. Both employed identity priming, where the prime and target are the same lexical item but differ in case (NEWS-news). The first experiment used only forward masking, while the prime in the second experiment was both preceded and followed by a mask (backward masking). In both studies, we find a significant behavioral effect of priming. Using MEG, we identified a component peaking approximately 225 ms post-onset of the target, whose latency was sensitive to repetition. These findings support the notion that properties of the MEG response index specific lexical processes and demonstrate that masked priming can be effectively combined with MEG to investigate the nature of lexical processing.
Keywords: Magnetoencephalography, MEG, Masked Priming, Backward Masking, Identity Priming, Immediate Repetition Priming
Introduction
Masked priming has proven to be a powerful psycholinguistic tool in probing lexical activation. Priming allows for the comparison of psychophysical responses at the initial stages of lexical access, avoiding the types of strategic cognitive processes thought to be involved in straightforward lexical decision experiments (Forster and Davis 1984; Forster 1998; Forster 1999; Forster et al. 2003; see Masson and Bodner 2003 for a critical review). Moreover, it has been shown that priming is possible even at very brief prime durations (roughly 30–60 ms), considerably minimizing the amount of controlled or conscious processing, and ideally, allowing for mostly automatic processes to influence decision to the target (see Forster 1998 for a recent assessment).
Behavioral masked priming studies have shown robust effects on reaction times to targets. For example, Forster and Davis (1984) found an identity priming effect. Primes were presented for 60 ms and followed by either the identical word (different case) or a different word. Both high and low frequency words showed this priming effect, and there was no interaction between frequency and priming. More recently, masked priming studies have investigated the morphological relation between primes and targets. For example, in French, Longtin et al. (2003) found priming for morphologically transparent (gaufrette-GAUFRE ‘wafer’-‘waffle’), opaque (fauvette-FAUVE ‘warbler’-‘wildcat’) and pseudo-derived (baguette-BAGUE ‘little stick’-‘ring’) pairs when primes were presented for 46 ms. Rastle et al. (2000) and Rastle et al. (2004) found a similar pattern of results in English for masked primes at durations of 43 ms and 42 ms, respectively.
Despite the utility of priming tasks - and in particular masked priming - for studying automatic, unconscious lexical processing, it is important to note that even in these experiments, our only index of processing is manifested in a decision stage response. Electrophysiology, however, provides the potential for measuring pre-decision processes (see Pylkkänen and Marantz 2003). Thus, combining a psycholinguistic methodology that purportedly probes automatic lexical activation with a recording technique that indexes pre-decision responses should prove insightful for our understanding of lexical representations and the (temporal sequence of) processing stages involved in lexical access.
Given that prior studies suggest that properties of averaged MEG responses do, indeed, reflect specific subroutines in lexical processing (Embick et al. 2001; Pylkkänen et al. 2002; Beretta et al. 2005; Fiorentino and Poeppel 2007), there is reason to think that, when using priming designs concurrently with MEG, we may be able to identify how and when a particular prime-target relation influences the time course of processing a given target. As a matter of fact, previous MEG studies using overt immediate priming (Stockall and Marantz 2006) and medium distance repetition priming (Sekiguchi et al. 2000, 2001) have shown effects around 300–600 ms post-onset of the target. However, there has been no evidence reported in the literature to date establishing the neural correlates of masked priming using MEG.
In the EEG literature, there have been several recent reports showing evoked responses sensitive to masked priming. In a recent ERP study, Holcomb and Grainger (2006) tested word-form relatedness under masked conditions. Participants were presented with 500 ms of mask (‘########’), followed by 50 ms of prime, 20 ms of backward mask and subsequently the target. They tested three conditions: whole-word identity priming (table-TABLE), partial-identity priming (teble-TABLE) and unrelated prime-target pair (mouth-TABLE). They found a difference between the unrelated condition and the identity and partial-identity conditions at 150 ms post-onset of the target, and found three-way-differential patterns in the ERP evoked response from roughly 250 ms post-onset of the target through the N400. Morris et al. (2007), also using ERP, compared morphologically transparent (hunter-hunt), opaque (corner-corn) and orthographic overlap (scandal-scan) conditions, using primes of 50 ms. They found a significant difference in the amplitude of a component whose peak latency was around 250 ms across all three conditions. Lavric et al. (2007) tested prime-target pairs that were either morphologically transparent (hunter-HUNT), morphologically opaque (corner-CORN) or had no morphological relationship (brothel-BROTH). Primes were presented for 42ms between the forward mask (500 ms) and target. They found a reduction in N400 amplitude for the morphologically transparent and opaque conditions relative to the no morphological relationship condition, again providing further evidence that ERP evoked responses are sensitive to priming under masked conditions. Given that ERP studies seem to identify responses sensitive to priming, it reinforces the question whether or not an equivalent or related response is observable using MEG.
More generally, the use of MEG is motivated by the further fact that the added sensitivity of this methodology permits the investigation of within-subjects effects of the type at stake here. The ERP studies that have demonstrated sensitivity to masked priming (where it must be noted that the effects are sometimes in amplitude and sometimes latency) report grand average data. The experiments discussed here suggest that these effects can be observed at a single subject level, thereby extending the findings from the literature.
Assuming that MEG responses reflect aspects of lexical processing (in a practically useful manner), then the neuromagnetic signal should show sensitivity to masked priming. This link has not yet been unequivocally demonstrated, even with masked identity priming. Fujimaki et al. (2004) used MEG and tested cross-syllabary priming in Japanese under very brief prime durations. Primes and targets were presented vertically, just left of center. The prime was preceded by 1000 ms of mask and its duration was 70 ms. Primes were presented in the Hiragana syllabary, and the targets (same lexical item) were presented in the Katakana syllabary. Fujimaki et al. (2004) failed to find any difference when the MEG waveform for the repeated prime condition (prime and target are same lexical item, but from a different syllabary) was subtracted from the control condition (the prime is composed of pseudo-characters and the target is a real word in Katakana). There are two caveats that should be mentioned regarding this study, however. First, no robust behavioral effect was found across participants for the repeated condition. This makes interpreting the electrophysiological data more difficult. Second, it should be emphasized that the prime and target were not simply presented in a different case, as is commonly done. Instead, they were presented in different syllabaries. This is not equivalent to presenting prime-target pairs that only differ in case (stroke differences between Hiragana and Katakana are often significant), and consequently, could have led to the neutralization any priming effect that might have been found.1
Since behavioral data on identity priming are robust (Forster and Davis 1984; Forster et al. 2003) and, by all accounts, should yield facilitatory effects for repeated words, identity priming constitutes an ideal test of the fundamental assumption that priming should be reflected in the MEG signal. Thus, in the current study, we chose identity priming.
Previous MEG studies have identified a series of response components following the onset of a visual word that appear to index stages in lexical processes. Activation around 100–200 ms stimulus post-onset, originating from occipotemporal regions, has been shown to be sensitive to visual word form properties, such as letter-string length and perceptibility (Pammer et al. 2004; the type I and II responses reported in Tarkiainen et al. 1999; Cornelissen et al. 2003). Similarly, bilateral occipitotemporal components peaking around 130 ms (M130) and 170 ms (M170) have been observed in a number of MEG visual lexical decision studies, but typically do not systematically vary on properties thought to affect lexical access, such as word frequency, semantic properties, and priming (Stockall et al. 2004; cf. Assadollahi and Pulvermüller 2003). A subsequent component, the M250 has been argued to be sensitive to phonotactic probability (Stockall et al. 2004), and the M350 has been shown to be sensitive to lexical frequency (Embick et al. 2001), phonotactic probability (Pylkkänen et al. 2002), morphological family size (Pylkkänen et al. 2004) and the number of polysemes and homophones associated with a given lexical entry (Beretta et al. 2005). As a consequence, the time window of 100–350 ms provides the temporal boundary conditions within which we are looking for a systematic response modulation of the MEG temporal signal associated with stages of lexical access. Moreover, previous ERP experiments using masked priming with prime durations of roughly 50 ms have shown differences around 250 ms post-onset of the target (Holcomb and Grainger 2006; Morris et al. 2007). Therefore, we too, might expect to find differences around this latency.
We present two masked identity-priming experiments and identify a component peaking approximately 225 ms post-onset of the target whose latency is sensitive to repetition. Our data are consistent with recent ERP findings using masked priming (Holcomb and Grainger 2006; Morris et al. 2007) that show response modulation at approximately the same latency. This component is consistent across two experiments, which employ slightly different masking paradigms (forward mask only; forward and backward mask).
There are two reasons why we tested two paradigms. First, both paradigms are used in the psycholinguistic literature to argue for claims about lexical access and structure (Forster and Davis 1984; Forster 1998; Forster 1999; Forster et al. 2003; Dehaene et al. 2004; Holcomb and Grainger 2006; Morris et al. 2007). Second, we hoped to determine that a neurophysiological result would be robust and replicable under two slightly different elicitation conditions.2 Our findings lead to at least two conclusions. First, the evoked MEG response indexes and is demonstrably sensitive to specific processes involved in lexical access and representation. Ultimately, this provides a window into the neurocomputational mechanisms involved in word recognition and linguistic processing. Second, from a methodological perspective, the powerful psycholinguistic tool of masked priming can be effectively combined with MEG, an electrophysiological recording measure that has been shown to be sensitive to an array of lexical processes.
MATERIALS AND METHODS
Participants
Fourteen native monolingual American English speakers participated in Experiment 1 (9 female; mean age: 20.5 yrs). Fourteen subjects participated in Experiment 2 (6 female; mean age: 22.5 yrs). Experiment 2 subjects were naïve and did not participate in the first experiment. All subjects tested strongly right-handed on the Edinburgh Handedness Inventory (Oldfield 1971). Subjects were compensated $10/hr. for their participation. Each session lasted approximately 1 ½ to 2 hours. Participants provided informed consent.
Stimuli
The two experiments contrast words that are either primed by the identical word in upper case or primed by a different word in upper case. Seventy-six words were in the repeated condition (VIDEO-video). An additional 76 words were in the non-repeated condition (VIDEO-motor). The word targets in both conditions were controlled on log frequency (repeated: 1.76; nonrepeated: 1.76; p = 0.81; Collins Cobuild, 320 million words; for Cobuild resources, see http://www.cobuild.collins.co.uk), length (repeated: 4.21 letters/word; nonrepeated: 4.21 letters/word) and syllabicity (repeated: 1.1 syllables/word; nonrepeated: 1.1 syllables/word).
The word targets used were presented in a previous norming lexical decision study. In the norming study, no difference in reaction time (paired, two-tailed t-test, t(20) = −0.172, p = 0.865) or accuracy (paired, two-tailed t-test, t(20) = 0.165, p = 0.871) was found (Fiorentino 2005). One hundred fifty two non-words preceded by real word primes acted as fillers (CODE-dess). Non-words were matched to words on length and syllabicity. The primes for words (targets and controls) were also matched on frequency with the primes for non-words (words = 1.76; non-words = 1.85; p = 0.33). All primes were real words, such that lexicality (and frequency) of the prime did not predict lexicality of the target. Items were presented in pseudorandom order.
Procedure and recording
Experiment 1
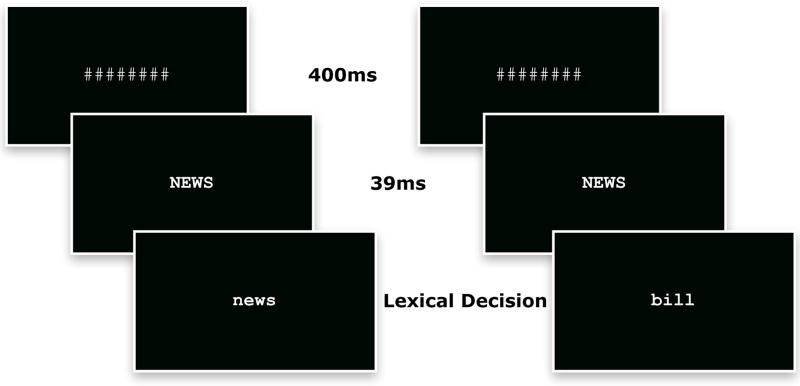
Participants lay supine in a magnetically shielded room, with low ambient lighting. Neuromagnetic fields were measured using a 160-channel whole head axial gradiometer MEG system (Kanazawa Institute of Technology, Kanazawa, Japan). Figure 1 illustrates the trial structure. A trial consisted of a mask (i.e., ‘########’) for 400ms, the prime in upper case (e.g., MOUSE) for 39 ms, immediately followed by the target in lower case that was either the same as the prime (mouse), different from the prime (chill), or a non-word (clurg). The change in case from the prime to the target was done to ensure that the stimuli were physically distinct and required access to an appropriate (likely linguistic) representation. The target remained on the display until a lexical decision was made. If the participant failed to respond within 2500 ms, the current trial terminated and the subsequent trial began. The inter-trial interval pseudo-randomly varied between 400 ms and 759 ms. The items were presented in Courier New font, in which upper and lower case items are quite visually distinct. The text was yellow and against a black background (visual angle: 0.67° horizontal and vertical per character; 2.83° horizontal per average word). Stimuli presentation was delivered using DMDX (Forster and Forster 2003). In post-experimental debriefing, no participants reported awareness of the prime.
FIGURE 1.
Trial structure used in Experiment 1. The mask remained on the screen for 400 ms, immediately followed by the prime for 39 ms, immediately followed by the target, which remained on the display until a lexical decision was made. The repeated condition is presented on the left, the nonrepeated on the right.
Experiment 2
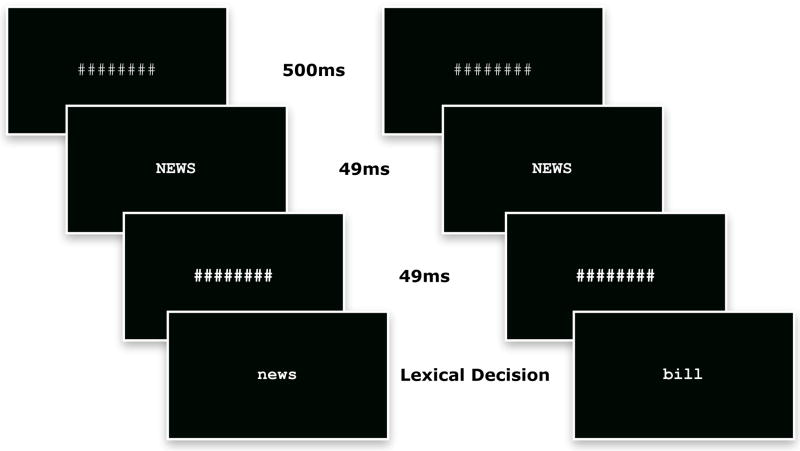
A similar procedure to Experiment 1 was used with the addition of a mask following the prime (a backward mask). The trial structure, presented in Figure 2, was for participants to see 500 ms of mask (‘########’), immediately followed by the prime (i.e., NEWS) with a duration of 49 ms, immediately followed by 49 ms of backward mask (‘#######’), immediately followed by the target (i.e., news, bill).
FIGURE 2.
Trial structure used in Experiment 2. The mask remained on the screen for 500 ms, immediately followed by the prime for 49 ms, immediately followed by the backward mask for 49 ms, immediately followed by the target, which remained on the display until a lexical decision was made or timeout. The repeated condition is presented on the left, the nonrepeated on the right.
Data analysis
The MEG data were sampled at 1 kHz (recording bandwidth DC to 100 Hz, online low pass filter; 60 Hz online notch filter). Offline, the data were noise reduced using a multi-shift PCA noise reduction algorithm (Cheveigné and Simon 2007), and a bandpass filter from 0.03 Hz–14 Hz was applied subsequent to selective averaging by condition. All trials that were either incorrect or associated with response times greater than 2.5 standard deviations from the individual’s mean were not included in MEG data analysis (Experiment 1: 7.3% of the total trials; Experiment 2: 7.1%; note: no trials were 2.5 standard deviations faster than the individual’s mean in either experiment). All remaining trials were averaged by condition. Across conditions and participants, a robust peak was elicited approximately 225 ms post-onset of the target stimulus, as in Figure 3. We selected ten channels from only the source (outgoing magnetic field) in the left hemisphere of this component for the analysis, given that the sink (ingoing magnetic field) was not clearly visible across participants due to limitations of the sensor configuration as well as subjects’ head position in the scanner. The sensors were selected on a participant-by-participant basis, analyzing the ten sensors with the strongest magnetic field strength. The same sensors were used across conditions for any given participant. The peak latency and amplitude of the components in the RMS (root mean squared) averaged waveform were carried forward for statistical analysis.
FIGURE 3.
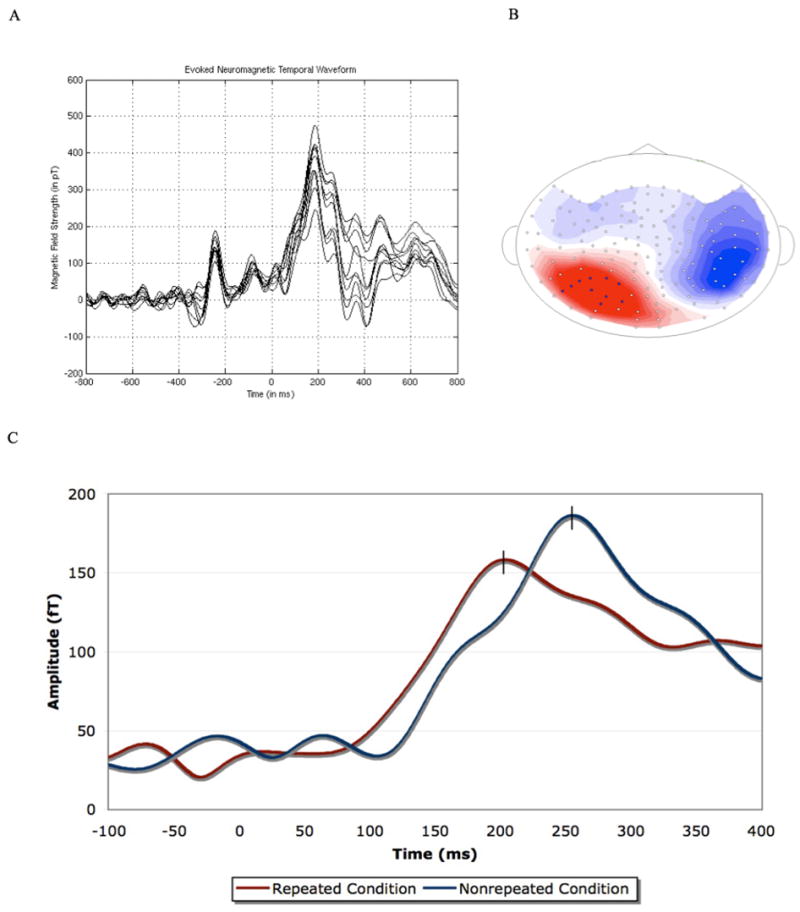
(A) An overlay plot of ten channels (those selected) from the source of the component (left posterior). Time 0 indicates the onset of the target. Because they are all taken from an outgoing magnetic field (source), only the positive components of the waveform are present. The data is from one representative subject. (B) The magnetic field distribution of the one robust component elicited in both experiments from one participant. The magnetic field distribution presented is that of the repeated condition (the red coloration indicates an outgoing magnetic field (source), while the blue coloration indicates an incoming magnetic field (sink); note that the visible sink and visible source are not generated from the same dipole as they are in different hemispheres). (C) RMS waveform of the one robust identifiable peak identified after the onset of the target. Time 0 indicates the onset of the target. Data is from representative subject. Note that the apparent difference in amplitude was not reliable across participants, while the difference in latency was.
RESULTS
Experiment 1
Psychophysics
Reaction time data showed a robust effect of repetition (paired, two-tailed t-test, t(13) = 2.20, p < 0.001). There was no reliable difference in the overall accuracy between the two conditions (paired, two-tailed t-test, t(13) = 1.66, p = 0.13). As predicted, repeated words are responded to more quickly even at very brief prime durations (Forster and Davis 1984; Forster 1998).
MEG Results
Having shown that the psychophysical measure yields a robust priming effect, we turn to the analysis of the MEG response to determine if there exists a reliable component sensitive to repetition priming under masked conditions. Figure 3 illustrates that in an RMS-average waveform analysis, a large peak at roughly 225 ms post-onset of the target was observed. This was found consistently across participants and conditions. As predicted, repeated words elicited an earlier peak latency than non-repeated words (repeated: 214 ms; nonrepeated: 228 ms; paired, two-tailed t-test, t(13) = 2.20, p < 0.05). This is shown in Figure 3C. The amplitude of this peak showed no sensitivity to repetition (repeated: 192 fT; nonrepeated: 191 fT; paired, two-tailed t-test, t(13) = 0.15, p = 0.9). Thus, the component whose peak-latency was centered roughly 225 ms post-onset of the target is sensitive to repetition priming under masking conditions. Its magnetic field contour, presented in Figure 3B (though not its timing), is similar to that of the M170, which is an occipitotemporal components that peak around 130–200 ms and sensitive to various properties of visual word forms such as word length (Pylkkänen and Marantz 2003; Stockall et al. 2004). While the previous MEG and ERP literature on single word recognition (i.e., not under masked conditions) might also lead us to predict differences in later time windows, roughly 350 ms to 500 ms post-onset of the target, no reliable component was found in time windows beyond the component elicited approximately 225 ms post-onset of the target across participants. The peak latencies and amplitudes for this component per participant are presented in Table 2.
TABLE 2.
Latency (in ms) and Amplitude (in fT) by subject for both repeated and nonrepeated conditions for Experiment 1.
| Subject | Repeated Condition | Nonrepeated Condition | ||
|---|---|---|---|---|
| Latency | Amplitude | Latency | Amplitude | |
| 1 | 215 | 121 | 210 | 100 |
| 2 | 292 | 188 | 276 | 229 |
| 3 | 215 | 157 | 260 | 156 |
| 4 | 235 | 217 | 239 | 201 |
| 5 | 201 | 161 | 253 | 186 |
| 6 | 206 | 154 | 278 | 145 |
| 7 | 253 | 143 | 275 | 149 |
| 8 | 203 | 107 | 194 | 109 |
| 9 | 167 | 138 | 181 | 168 |
| 10 | 204 | 259 | 210 | 240 |
| 11 | 224 | 235 | 219 | 169 |
| 12 | 183 | 429 | 187 | 420 |
| 13 | 179 | 141 | 192 | 152 |
| 14 | 217 | 242 | 228 | 253 |
| Mean | 214 | 192 | 229 | 191 |
| Standard Deviation | 31.6 | 83 | 35 | 79.7 |
Discussion
In the first experiment, we identified a component sensitive to repetition priming under masking conditions, whose peak latency was roughly 225 ms post-onset of the target, replicating the findings from the ERP literature, where masked primes with a duration of roughly 50 ms elicit differences around 250 ms post-onset of the target (Holcomb and Grainger 2006; Morris et al. 2007). Specifically, the peak latency of the component was earlier when the prime and target were identical (repeated condition) than when the prime and target were different words (non-repeated condition). We suggest these findings show that it is possible to identify neural correlates of retrieval sensitive to priming using MEG, that MEG and masked priming can be fruitfully combined, and finally, that we have, at least tentatively, identified a dependent measure to test further prime-target relations in subsequent studies. The pattern of responses in our MEG dataset, following the presentation of the target word, did not resemble that typically observed in MEG single word data (Embick et al. 2001; Pylkkänen et al. 2002; Pylkkänen and Marantz 2003; Stockall et al. 2004), in which at least three components are generally found: M170, M250 and M350. The reason for this could be because there was no pause between the visual stimuli (mask-prime-target). The response does, however, closely resemble that of the other MEG study of masked priming (Fujimaki et al. 2004). In this study, they find one large prominent peak following the onset of the target. The results of the current study show an MEG component that reflects repetition priming whose latency is roughly 225 ms post-onset of the target, consistent with the ERP results, such as those in Morris et al. (2007).
Experiment 2
To ensure that this component is robust and reliable, we tested an alternative, commonly used masked priming paradigm: backward masking (Dehaene et al. 2004; Holcomb and Grainger 2006; among others). We adjusted the priming paradigm slightly by inserting a backward mask (“########”) between the prime and target, as illustrated in Figure 2, and repeated the experiment with a new set of participants. This was done to ensure our effect would remain under this altered priming paradigm (see Forster 1998; Forster et al. 2003 for discussion of the these alternative paradigms in the psycholinguistic literature).
Psychophysics
Psychophysically, words in the repeated condition were responded to more quickly than words in the nonrepeated condition (Paired, two-tailed t-test, t(13) = 4.62, p < 0.0005). There was no difference in accuracy (Paired, two-tailed t-test, t(13) = 1.06, p = 0.31). Again, as predicted, repeated words were responded to more quickly under masked conditions than nonrepeated words. In both Experiment 1 (forward mask only) and Experiment 2 (forward and backward mask), we find a robust behavioral result.
Thus, under the prediction that MEG reflects differences in lexical activation, and based on our findings from Experiment 1, we should once again elicit an earlier peak latency in the component whose peak latency is around 225 ms.
MEG Results
In the RMS-averaged MEG responses, consistently across participants and conditions, we again observed a large response whose latency was approximately 225 ms post-onset of the target.
Based on the results of the first experiment, we predict that the component centered approximately 225 ms post-onset of the target should show a significant latency effect in favor of the repeated words. Indeed, repeated words elicited a significantly faster latency than nonrepeated words (repeated: 223 ms; nonrepeated: 236 ms; paired, two-tailed t-test t(13) = 2.19, p < 0.05). The peak latencies and amplitudes for this component per participant in Experiment 2 are presented in Table 4. Again, no significant amplitude difference was found (repeated: 167 fT; nonrepeated: 167 fT; paired, two-tailed t-test, t(13) = −0.25, p = 0.8) across conditions. In this dataset, an earlier peak was also evident, appearing around 120 ms post-onset; however, this component showed no significant differences in latency (repeated: 121ms; nonrepeated: 121 ms; paired, two-tailed t-test, t(13) = −0.50, p = 0.62) or amplitude (repeated: 122 fT; nonrepeated: 122 fT; paired, two-tailed t-test, t(13)=0.33, p = 0.74) across conditions. Also, akin to the findings from the first experiment, the peak around 225 ms post-onset of the target had a magnetic field contour similar to that of an M170. Again, no component was reliably identified in later time windows.
Table 4.
Latency (in ms) and Amplitude (in fT) by subject for both repeated and nonrepeated conditions for Experiment 2.
| Subject | Repeated Condition | Nonrepeated Condition | ||
|---|---|---|---|---|
| Latency | Amplitude | Latency | Amplitude | |
| 1 | 197 | 177 | 206 | 149 |
| 2 | 192 | 327 | 194 | 296 |
| 3 | 210 | 91 | 210 | 88 |
| 4 | 280 | 128 | 264 | 199 |
| 5 | 213 | 150 | 257 | 211 |
| 6 | 203 | 184 | 210 | 221 |
| 7 | 217 | 113 | 244 | 86 |
| 8 | 179 | 239 | 179 | 233 |
| 9 | 285 | 146 | 277 | 179 |
| 10 | 235 | 191 | 258 | 194 |
| 11 | 220 | 111 | 266 | 160 |
| 12 | 276 | 148 | 260 | 117 |
| 13 | 227 | 138 | 267 | 76 |
| 14 | 194 | 162 | 208 | 142 |
| Mean | 223 | 167 | 236 | 167 |
| Standard Deviation | 34.2 | 60.1 | 32.8 | 63.2 |
Discussion
Despite the addition of a backward mask in Experiment 2, we again identified a reliable latency difference in the MEG signal around 225 ms post-onset of the target that patterns with reaction times, with an earlier peak latency for repeated words compared to nonrepeated words. This demonstrates the robustness and replicability of the component and argues for its use as a useful dependent variable in future experiments testing different prime-target relations. Similar to the first experiment, the latency, but not amplitude of this component, is sensitive to repetition priming under masked conditions. The utility of the response is supported by the finding that the means, standard deviations and directionality of the responses are very similar across experiments.
Given that the magnetic field pattern of the response observed here is consistent with that of an M170, based on other experiments using comparable materials in single-word presentation (Embick et al. 2001; Pylkkänen et al. 2002; Stockall et al. 2004; Beretta et al. 2005; Fiorentino and Poeppel 2007; see Pylkkänen and Marantz 2003 for review), there are reasons to suspect that this component is indeed the M170. On the other hand, its peak latency is fairly late for a canonical M170 response (the M170 is typically elicited approximately 150–200 ms following the onset of a visual word. Moreover, in full repetition (identity) priming studies, the materials conflate orthographic, phonological, morphological and semantic properties, making it difficult to conclude – based on that particular paradigm alone – which property or properties of the prime-target relation the identified component is sensitive to. Consequently, assigning this particular activation pattern to a standard component can only be tentative, at best. While the current study provides a proof of principle that this component can be elicited, we remain agnostic as to whether this component actually reflects the M170 source. In short, we do not want to conclude that a new component has been discovered; on the other hand, we cannot conclusively claim that this is the M170 because of the nature of the prime-target stimuli. Future research is required to disentangle these confounds and ultimately relate the observed component to those identified in the literature both with respect to its sensitivity and underlying functional neuroanatomy.
GENERAL DISCUSSION
The experiments summarized here had two aims: First, we wanted to determine whether MEG and masked priming could be productively combined. Finding a reliable difference in the MEG response sensitive to repetition under masked conditions yields a dependent variable for use in future experiments testing a variety of prime-target relations. Second, we wanted to further illuminate the question to what extent the MEG evoked field reflects (independently motivated) processes involved in lexical access (Embick et al. 2001; Pylkkänen et al. 2002; Pylkkänen and Marantz 2003; Beretta et al. 2005; Fiorentino and Poeppel 2007). Therefore, we ran two masked priming studies using MEG. We reasoned that by combining a psycholinguistic methodology sensitive to word structure (i.e., masked priming) with an electrophysiological technique that allows us to tap into pre-decision lexical processes, we can better understand the time course of lexical access and the nature of the implicated representations. We conducted two experiments, the first with only a forward mask and the second with both a forward and backward mask, and we identified a reliable latency difference roughly 225 ms post onset of the target that was sensitive to repetition. The timing of the response we identified is consistent with recent ERP findings that used masked priming with brief prime durations (roughly 50 ms). The identification of this component establishes that the effect of masked identity priming can be demonstrated in the evoked field, thus allowing for more linguistically interesting prime-target relations to be investigated, with the ultimate goal of better understanding the nature of linguistic representations.
TABLE 1.
Reaction times and accuracy for the repeated word and nonrepeated word conditions in Experiment 1.
| Condition | Reaction Time (ms) | Accuracy (%) |
|---|---|---|
| Repeated Words | 672 | 97.3 |
| Nonrepeated Words | 703 | 96.4 |
| Difference | −31* | +0.9 |
denotes p < 0.001.
TABLE 3.
Reaction times and accuracy for the repeated word and nonrepeated word conditions in Experiment 2.
| Condition | Reaction Time (ms) | Accuracy (%) |
|---|---|---|
| Repeated Words | 625 | 98 |
| Nonrepeated Words | 665 | 97.4 |
| Difference | −40* | +0.6 |
denotes p < 0.0005.
Acknowledgments
This project was supported by NIH DC R01 05660 to DP. We thank Jeff Walker for excellent technical assistance and William J. Idsardi, Diogo Almeida and two anonymous reviewers for numerous comments on the project and editorial suggestions on the manuscript.
Footnotes
It should be noted, however, that some cases of cross-script priming have been found in Japanese. For example, Hino et al. (2003) found priming between Kanji and Katakana.
Researchers disagree on which design is most suitable for investigating lexical access (for discussion of methodological concerns, see Forster 1999; Masson and Isaak 1999; Forster and Forster 2003; Masson and Bodner 2003). This study is not designed to adjudicate between the differing options, but instead to show that both paradigms can be used productively in combination with the advantages that MEG offers.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assadollahi R, Pulvermüller F. Early Influences of Word Length and Frequency: A Group Study Using Meg. NeuroReport. 2003;14:1183–1187. doi: 10.1097/00001756-200306110-00016. [DOI] [PubMed] [Google Scholar]
- Beretta A, Fiorentino R, Poeppel D. The Effects of Homonymy and Polysemy on Lexical Access: An MEG Study. Cognitive Brain Research. 2005;24:57–65. doi: 10.1016/j.cogbrainres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Cheveigné Ad, Simon JZ. Denoising Based on Time-Shift PCA. Journal of Neuroscience Methods. 2007;165:297–305. doi: 10.1016/j.jneumeth.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen P, Tarkiainen A, Helenius P, Salmelin R. Cortical Effects of Shifting Letter Position in Letter Strings of Varying Length. Journal of Cognitive Neuroscience. 2003;15:731–746. doi: 10.1162/089892903322307447. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Bihan DL, Cohen L. Letter Binding and Invariant Recognition of Masked Words: Behavioral and Neuroimaging Evidence. Psychological Science. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Embick D, Hackl M, Schaeffer J, Kelepir M, Marantz A. A Magnetoencephalographic Component Whose Latency Reflects Lexical Frequency. Cognitive Brain Research. 2001;10:345–348. doi: 10.1016/s0926-6410(00)00053-7. [DOI] [PubMed] [Google Scholar]
- Fiorentino R. Masked Priming of Compound Constituents: Implications for Morphological Decomposition. Presented at the 11th AMLaP Conference; Ghent, Belgium. 2005. [Google Scholar]
- Fiorentino R, Poeppel D. Compound Words and Structure in the Lexicon. Language and Cognitive Processes. 2007;22:953–1000. [Google Scholar]
- Forster K. Pros and Cons of Masked Priming. Journal of Psycholinguistic Research. 1998;27:203–233. doi: 10.1023/a:1023202116609. [DOI] [PubMed] [Google Scholar]
- Forster KI. The Microgenesis of Priming Effects in Lexical Access. Brain and Language. 1999;68:5–15. doi: 10.1006/brln.1999.2078. [DOI] [PubMed] [Google Scholar]
- Forster KI, Davis C. Repetition Priming and Frequency Attenuation in Lexical Access. Journal of Experimental Psychology: Learning, Memory and Cognition. 1984;10:680–698. [Google Scholar]
- Forster KI, Forster JC. DMDX: A Windows Display Program with Millisecond Accuracy. Behavior Research Methods, Instruments & Computers. 2003;35:116–124. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- Forster KI, Mohan K, Hector J. The Mechanics of Masked Priming. In: Kinoshita S, Lupker SJ, editors. Masked Priming: The State of the Art. New York: Psychology Press; 2003. pp. 3–37. [Google Scholar]
- Fujimaki N, Hayakawa T, Munetsuna S, Matani A. Magnetic Responses to Visually Presented Words with Masked Repetition Priming. Presented at the 13th Annual Conference on Biomagnetism; Boston, MA. 2004. [Google Scholar]
- Hino Y, Lupker SJ, Ogawa T, Sears CR. Masked Repetition Priming and Word Frequency Effects across Different Types of Japanese Scripts: An Examination of the Lexical Activation Account. Journal of Memory and Language. 2003;48:33–66. [Google Scholar]
- Holcomb PJ, Grainger J. On the Time Course of Visual Word Recognition: An Event-Related Potential Investigation Using Masked Repetition Priming. Journal of Cognitive Neuroscience. 2006;18:1631–1643. doi: 10.1162/jocn.2006.18.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavric A, Clapp A, Rastle K. ERP Evidence of Morphological Analysis from Orthography: A Masked Priming Study. Journal of Cognitive Neuroscience. 2007;19:866–877. doi: 10.1162/jocn.2007.19.5.866. [DOI] [PubMed] [Google Scholar]
- Longtin CM, Segui J, Hallé PA. Morphological Priming without Morphological Relationship. Language and Cognitive Processes. 2003;18:313–334. [Google Scholar]
- Masson MEJ, Bodner GE. A Retrospective View of Masked Priming: Toward a Uniform Account of Masked and Long-Term Repetition Priming. In: Kinoshita S, Lupker SJ, editors. Masked Priming: The State of the Art. New York: Psychology Press; 2003. pp. 57–94. [Google Scholar]
- Masson MJ, Isaak MI. Masked Priming of Words and Nonwords in a Naming Task: Further Evidence for a Nonlexical Basis for Priming. Memory and Cognition. 1999;27:399–412. doi: 10.3758/bf03211536. [DOI] [PubMed] [Google Scholar]
- Morris J, Frank T, Grainger J, Holcomb PJ. Semantic Transparency and Masked Morphological Priming: An ERP Investigation. Psychophysiology. 2007;44:506–521. doi: 10.1111/j.1469-8986.2007.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. Assessment and Analysis of Handedness: Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pammer K, Hansen PC, Kringelbach ML, Holliday I, Barnes G, Hillebrand A, Singh KD, Cornelissen PL. Visual Word Recognition: The First Half Second. NeuroImage. 2004;22:1819–1825. doi: 10.1016/j.neuroimage.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Pylkkänen L, Feintuch S, Hopkins E, Marantz A. Neural Correlates of the Effects of Morphological Family Frequency and Family Size: An MEG Study. Cognition. 2004;91:B35–B45. doi: 10.1016/j.cognition.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Pylkkänen L, Marantz A. Tracking the Time Course of Word Recognition with MEG. Trends in Cognitive Sciences. 2003;7:187–189. doi: 10.1016/s1364-6613(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Pylkkänen L, Stringfellow A, Marantz A. Neuromagnetic Evidence for the Timing of Lexical Activation: An MEG Component Sensitive to Phonotactic Probability but Not to Neighborhood Density. Brain and Language. 2002;81:666–678. doi: 10.1006/brln.2001.2555. [DOI] [PubMed] [Google Scholar]
- Rastle K, Davis MH, Marslen-Wilson WD, Tyler LK. Morphological and Semantic Effects in Visual Word Recognition: A Time-Course Study. Language and Cognitive Processes. 2000;15:507–537. [Google Scholar]
- Rastle K, Davis MH, New B. The Broth in My Brother’s Brothel: Morpho-Orthographic Segmentation in Visual Word Recognition. Psychonomic Bulletin & Review. 2004;11:1090–1098. doi: 10.3758/bf03196742. [DOI] [PubMed] [Google Scholar]
- Sekiguchi T, Koyama S, Kakigi R. The Effect of Word Repetition on Evoked Magnetic Responses in the Human Brain. Japanese Psychological Research. 2000;42:3–14. [Google Scholar]
- Sekiguchi T, Koyama S, Kakigi R. The Effect of Stimulus Repetition on Cortical Magnetic Responses Evoked by Words and Nonwords. NeuroImage. 2001;14:118–128. doi: 10.1006/nimg.2001.0774. [DOI] [PubMed] [Google Scholar]
- Stockall L, Marantz A. A Single Route, Full Decomposition Model of Morphological Complexity: MEG Evidence. The Mental Lexicon. 2006;1:85–123. [Google Scholar]
- Stockall L, Stringfellow A, Marantz A. The Precise Time Course of Lexical Activation: MEG Measurements of the Effects of Frequency, Probability and Density in Lexical Decision. Brain and Language. 2004;90:88–94. doi: 10.1016/S0093-934X(03)00422-X. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of Letter String Perception in the Human Occipitotemporal Cortex. Brain. 1999;122:2119–2131. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]