Abstract
MicroRNAs (miRNAs) are members of a family of non-coding RNAs of 8-24 nucleotide RNA molecules that regulate target mRNAs. The first miRNAs, lin-4 and let-7, were first discovered in the year 1993 by Ambros, Ruvkun, and co-workers while studying development in Caenorhabditis elegans. miRNAs can play vital functions form C. elegans to higher vertebrates by typical Watson-Crick base pairing to specific mRNAs to regulate the expression of a specific gene. It has been well established that multicellular eukaryotes utilize miRNAs to regulate many biological processes such as embryonic development, proliferation, differentiation, and cell death. Recent studies have shown that miRNAs may provide new insight in cancer research. A recent study demonstrated that more than 50% of miRNA genes are located in fragile sites and cancer-associated genomic regions, suggesting that miRNAs may play a more important role in the pathogenesis of human cancers. Exploiting the emerging knowledge of miRNAs for the development of new human therapeutic applications will be important. Recent studies suggest that miRNA expression profiling can be correlated with disease pathogenesis and prognosis, and may ultimately be useful in the management of human cancer. In this review, we focus on how miRNAs regulate tumorigenesis by acting as oncogenes and anti-oncogenes in higher eukaryotes.
Keywords: C. elegans, cancer, miRNA, oncomirs, non-coding
Background
Cancer is caused by misappropriate expression of genes and is one of the most lethal diseases affecting human population. The first proof that miRNAs are involved in cancer came from the finding that miR-15a and miR-16-1 are down-regulated or deleted in most patients with chronic lymphocytic leukemia (CLL) [1]. The use of miRNA microarrays made possible large profiling studies in cancer patients, confirming that miRNAs are differentially expressed in normal and tumor samples [2].
In this review, we briefly described miRNA biogenesis and the principle approaches for studying the function of miRNAs in cancers. Furthermore, we focused on miRNAs as oncogenes and tumor suppressors by outlining the evidence for the involvement of miRNAs in numerous human cancers, with several specific examples from both experimental and clinical analysis. To exemplify how miRNA can be useful, potential application of miRNAs as biomarkers, diagnosis, and potential therapeutic tools of human cancers are discussed. Finally, we provide an overview of some of the past and potential future aspects and present a broad overview of this rapidly growing field.
MicroRNA biogenesis and mechanism
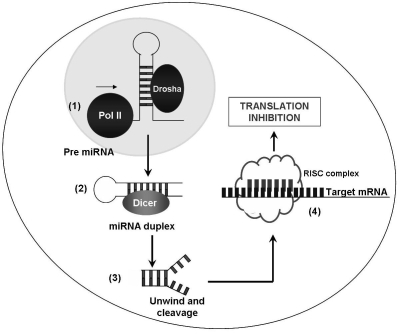
miRNAs are encoded in the genome and are transcribed by RNA polymerase II (pol II) as long precursor transcripts, which are known as primary miRNAs (pri-miRNAs) of several kilobases in length [3]. Mature miRNAs are generated from pri-miRNAs by sequential processing steps. The pri-miRNAs are initially recognized by the microprocessor complex in the nucleus, whose core components are the RNase-III enzyme Drosha and its binding partner DGCR8 [4-7]. The microprocessor complex excises the stem-loop hairpin structure that contains the miRNA, a 60-80 nucleotide intermediate termed precursor miRNA (pre-miRNA). The pre-miRNA is recognized by the nuclear export factor Exportin-5, which transports it to the cytoplasm [8-10]. Pre-miRNAs are rapidly exported to the cytoplasm by the nuclear export factor exportin 5 which uses Ran-GTP as a cofactor [8-10]. Further cytoplasmic processing by dicer, a second RNase III enzyme named Dicer performs a second cleavage to generate double-stranded 18-24 nucleotide-long [11-14]. One of these two strands - the guide strand - is incorporated in an ATP-independent manner into a large protein complex known as the RNA-induced silencing complex, or RISC, which includes as core components the Argonaute proteins (Ago1 - 4 in humans) [15]. Only one strand of the miRNA duplex remains stably associated with RISC. This strand becomes the mature miRNA. The opposite strand, known as the passenger strand, is disposed of through two alternative mechanisms. When miRNAs are loaded into RISC containing Ago2, the only human Ago protein capable of cleaving target mRNAs, the passenger strand may be cleaved. Alternatively, RISC containing any Ago protein may remove the passenger strand via a bypass mechanism that does not require cleavage and likely involves duplex unwinding [16-18]. The miRNA guides the RISC complex to the target mRNA, which will then be subsequently cleaved or translationally silenced. The degree of complementarity between an miRNA and its target determines the mechanism of silencing ding on the degree of sequence complementarity of the miRNA to its target mRNA [19-21].
MicroRNA expression profiling
Currently, almost all of the miRNA-related studies on cancers based on the different expression profile of miRNAs in cancer cells vs. normal cells. Differential expression of the candidate miRNAs is a good approach to study the function of miRNAs in cancer pathogenesis. Knockdown or overexpression of a specific miRNA allows studying the specific roles of the miRNA in tumorigenesis. Several groups have studied the miRNA expression in cancer patients and found that miRNAs are differentially expressed in normal and tumor tissues [2]. There are several methods have been proposed to conduct this study, such as antisense inhibitors, transgenics, northern blot, real-time PCR and miRNA microarray.
For cancer studies, it is important to be able to compare the expression pattern of all known miRNAs between cancer cells and normal cells. Two-color fluorescence-based microarray technology (DNA microarray) has been widely used to detect gene expression simultaneously. miRNA microarray offers detection of multiple differentially expressed miRNAs in cancers, may become a technique in cancer epidemiology and early cancer detection. Murakami and colleagues have exploited this method to detect precursors and mature miRNAs in 25 pairs of hepatocellullar carcinoma and adjacent non-tumoral tissue, and nine chronic-hepatitis tissues [22]. Their analysis showed a small number of miRNAs associated with differentiation state of the tumors, proposing that these miRNAs might contribute to both oncogenesis and loss of differentiation [22]. Microarray profiling was also used to study miRNAs that are differentially expressed in breast cancer. Researchers have reported results from 76 breast-cancer patients and ten normal breast samples and found 29 miRNAs, the expression of which is significantly deregulated in tumors [23]. A set of 15 miRNAs correctly predicted the nature of the breast-cancer sample analyzed with 100% accuracy. The highly up or downregulated miRNAs were miR-125b, miR-145, miR-21 and miR-155. Moreover, the expression of miRNAs was correlated with specific breast-cancer pathological features such as estrogen-receptor status, tumor stage, vascular invasion and proliferative index [23]. Overall, these studies have evidenced the existence of miRNA signatures that are tissue and tumor-specific and allow us to classify, diagnose and, in some cases, predict outcome accurately in cancer patients.
microRNAs as tumor suppressor genes
In oncogenesis, some miRNAs are downregulated in cancerous cells. These types of miRNAs are considered tumor suppressor genes (Table 1 in supplementary material). Tumor suppressor miRNAs generally prevent tumor development by negatively inhibiting oncogenes and/or genes that control cell differentiation or apoptosis. At present, various miRNAs are considered as tumor suppressor genes, for example, miR-15a and miR-16–1. Studies have shown that tumor suppressor role from frequent downregulation of miR-15a and miR-16-1 in CLL (Chronic lymphocytic leukemia) patients [1]. These two miRNAs are located in a commonly deleted area in CLL and both miR-15a and miR-16-1 act as tumor suppressors by downregulating B-cell CLL/lymphoma 2 (BCL2), a gene that is often upregulated in CLL [24,25]. Therefore, downregulation of miR-15a and miR-16-1, which is mainly due to a genetic deletion, might promote B-cell proliferation in CLL, a disease where the main feature is an abnormal neoplastic proliferation of B cells.
The second evidence that suggests a role of tumor suppressor for miRNAs came from the observation of frequent downregulation of let-7 in lung cancer [26]. In humans, let-7 is located at a chromosome region that is usually deleted in human cancers [27]. Let-7 was initially observed in C. elegans. let-7 is one of the beginning members of the miRNA family [28,29]. Improper expression of let-7 results in oncogenic loss of differentiation. Let-7 family members map to fragile sites associated with lung, breast, urothelial and cervical cancer [27].
microRNAs as oncogenes
miRNAs that are significantly over-expressed in tumors may be considered as oncogenes. All of them appear to function as oncogenes; nevertheless, only a few of them have been well characterized. These oncogene miRNAs, called “oncomirs”, usually promote tumor development by negatively inhibiting tumor suppressor genes and/or genes that control cell differentiation or apoptosis. Several studies and clinic analysis suggest that miRNAs may function as a novel class of oncogenes or tumor suppressor genes (Figure 1). miR-17-92 is a classic example for an oncogenic miRNA. mir-17-92 cluster is a miRNA polycistron located at chromosome 13q31-32, a region that is commonly amplified in B-cell lymphoma, was upregulated in 65% of the B-cell lymphoma samples examined [30,31]. Compared with normal tissues, the expression of mir-17-92 is significantly increased in several cancer types, including lung cancer and lymphomas, especially in their most aggressive forms, such as small-cell lung cancer and human B-cell lymphomas [30,31]. The miR-17-92 cluster also appears to enhance lung cancer cell growth [30]. To test whether this polycistron contributes to cancer formation, He and colleagues have used a well-characterized mouse model of B-cell lymphoma induced by the MYC gene. Using retroviral transduction, the authors overexpressed the miR-17-92 cluster in hematopoietic stem cells from mice that carry the MYC transgene. The enforced expression of the miR-17-92 cluster acted, together with MYC expression, to significantly accelerate the formation of lymphoid malignancies [31]. Co-expression of miR-17-19b, a truncated portion of miR-17-92, strongly accelerated lymphomagenesis [32]. Computational studies have shown that over 600 genes were targeted by miR-19a and miR-20, two members of miR-17-92 cluster [33,34]. Tumor suppressor genes PTEN (phosphatase and tensin homolog deleted on chromosome ten) and RB2 were predicted to be targeted by miR-17-92 cluster [35]. PTEN promotes apoptosis through the P13K-Akt-PKB pathway [32]. However, there was no biological evidence that PTEN and RB2 are right targets of the miR-17-92 cluster. The oncogenic role of the miR-17-92 cluster is further supported by profiling studies showing upregulation of this cluster in B-cell CLL [36] and in solid cancers [30,37]. It is logical to suggest that these miRNAs have distinct roles in distinct cell types, as was formerly proposed for well-known genes that encode tumor suppressors or oncogenes [38]. These studies suggest that mir-17-92 functions as an oncogene in humans and other animal models.
Figure 1.
The biogenesis and mechanism of human miRNA. The biogenesis of miRNA involves several enzymatic steps. Following transcription by RNA polymerase II (Pol II), capped and poly-adenylated primary miRNA transcripts (pri-miRNAs) are processed in the nucleus by the endonuclease Drosha into one or more premiRNAs (1). This pre-miRNA is exported from the nucleus to the cytoplasm and processed by another RNase enzyme called Dicer, which produces a transient 19-24 nucleotide duplex (2). Duplex is cleaved (3) and only one strand of the miRNA duplex (mature miRNA) is incorporated into into the RISC (RNA induced silencing complex), which retains only the single-stranded mature miRNA (4). This miRNA-programmed RISC negatively regulates the stability and/or translation of target mRNAs depending on the degree of complementary sites between the miRNA and its target.
Conclusion
Understanding of the role of miRNAs is allowing new insights on the molecular basis of cancers, and new biomarkers for cancer diagnoses and cancer therapy. The elucidation of how global miRNA expression contributes to phenotypic outcomes will be important to disease and/or signaling pathways that affect miRNA function as well as environmental or genetic factors that affect miRNA expression. Once this information is available, a rational approach can be taken to develop new therapeutic strategies that aim to correct inherited and acquired diseases. It will also be important to study combinatorial effects of multiple miRNAs on target gene expression [33]. The technological development in the field, particularly in the realm of bead-based flow cytometry, single molecule detection and massively parallel sequencing coupled with the miRAGE approach, may help launch an automatable, high-speed process for miRNA profiling in the near future [39-41]. Even though the discovery of miRNAs will probably change the landscape of cancer genetics, more work is required to understand the mechanism by which miRNAs contribute to cancer origin and progression.
Supplementary material
Acknowledgments
The work was partly supported by National Science Foundation of China, No. 90403010 and Scientific Research Projects of Inner Mongolia's Universities, No. NJZY07065.
References
- 1.Calin GA, et al. Proc Natl Acad Sci U S A. 2002;99:15524. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calin GA, Croce CM. Cancer Res. 2006;66:7390. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 3.Lee Y, et al. Embo J. 2004;23:4051. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denli AM, et al. Nature. 2004;432:231. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 5.Gregory RI, et al. Nature. 2004;432:235. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 6.Han J, et al. Genes Dev. 2004;18:3016. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landthaler M, et al. Curr Biol. 2004;14:2162. doi: 10.1016/j.cub.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Bohnsack MT, et al. RNA. 2004;10:185. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund E, et al. Science. 2004;303:95. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 10.Yi R, et al. Genes Dev. 2003;17:3011. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein E, et al. Nature. 2001;409:363. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 12.Grishok A, et al. Cell. 2001;106:23. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 13.Hutvagner G, et al. Science. 2001;293:834. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 14.Ketting RF, et al. Genes Dev. 2001;15:2654. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim VN. Mol Cells. 2005;19:1. [PubMed] [Google Scholar]
- 16.Gregory RI, et al. Cell. 2005;123:631. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Matranga C, et al. Cell. 2005;123:607. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 18.Rand TA, et al. Cell. 2005;123:621. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Doench JG, et al. Genes Dev. 2003;17:438. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutvagner G, Zamore PD. Science. 2002;297:2056. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Y, et al. Proc Natl Acad Sci U S A. 2003;100:9779. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami Y, et al. Oncogene. 2006;25:2537. doi: 10.1038/sj.onc.1209283. [DOI] [PubMed] [Google Scholar]
- 23.Iorio MV, et al. Cancer Res. 2005;65:7065. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 24.Cimmino A, et al. Proc Natl Acad Sci U S A. 2005;102:13944. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Beato M, et al. Blood. 2003;101:1220. doi: 10.1182/blood-2002-07-2009. [DOI] [PubMed] [Google Scholar]
- 26.Takamizawa J, et al. Cancer Res. 2004;64:3753. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 27.Calin GA, et al. Proc Natl Acad Sci U S A. 2004;101:2999. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambros V. Nature. 2004;431:350. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 29.Bartel DP. Cell. 2004;116:281. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 30.Hayashita Y, et al. Cancer Res. 2005;65:9628. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 31.He L, et al. Nature. 2005;435:828. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammond SM. Curr Opin Genet Dev. 2006;16:4. doi: 10.1016/j.gde.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Krek A, et al. Nat Genet. 2005;37:495. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 34.Lewis BP, et al. Cell. 2005;120:15. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 35.Lewis BP, et al. Cell. 2003;115:787. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 36.Calin GA, et al. N Engl J Med. 2005;353:1793. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 37.Kluiver J, et al. J Pathol. 2005;207:243. doi: 10.1002/path.1825. [DOI] [PubMed] [Google Scholar]
- 38.Calin G. Roum Arch Microbiol Immunol. 1992;51:271. [PubMed] [Google Scholar]
- 39.Lu J, et al. Nature. 2005;435:834. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 40.Neely LA, et al. Nat Methods. 2006;3:41. doi: 10.1038/nmeth825. [DOI] [PubMed] [Google Scholar]
- 41.Service RF. Science. 2006;311:1544. doi: 10.1126/science.311.5767.1544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.