Abstract
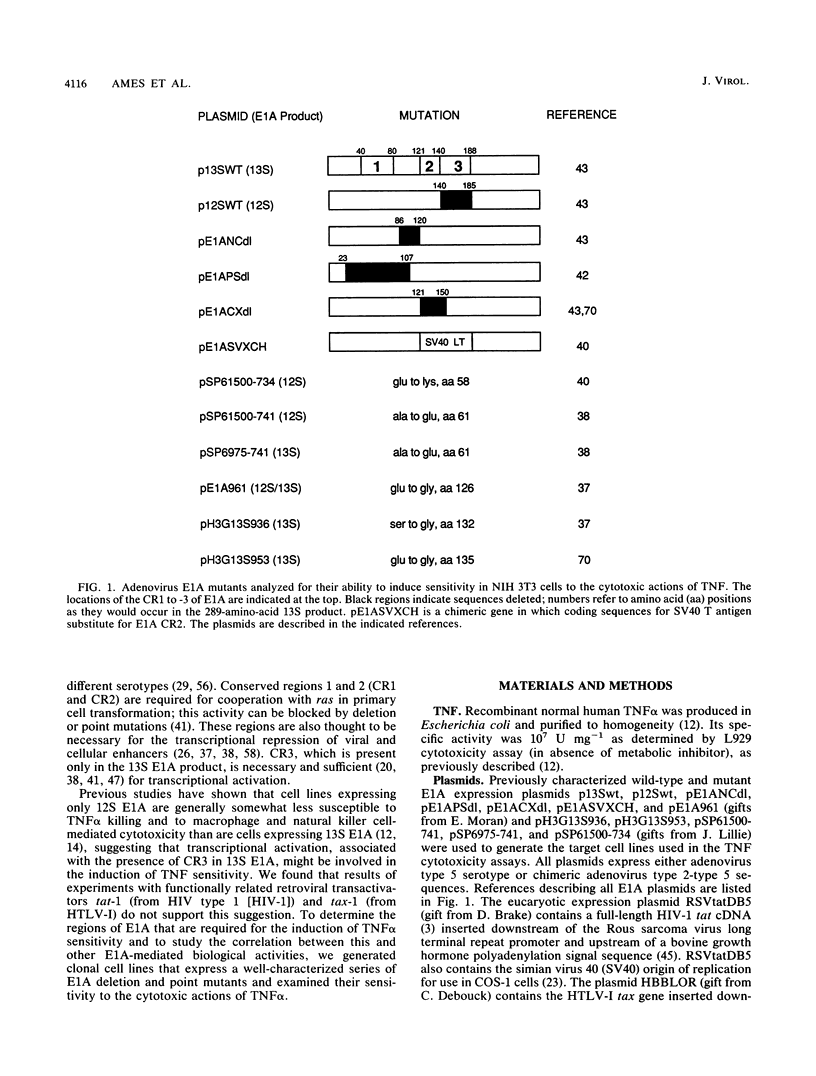
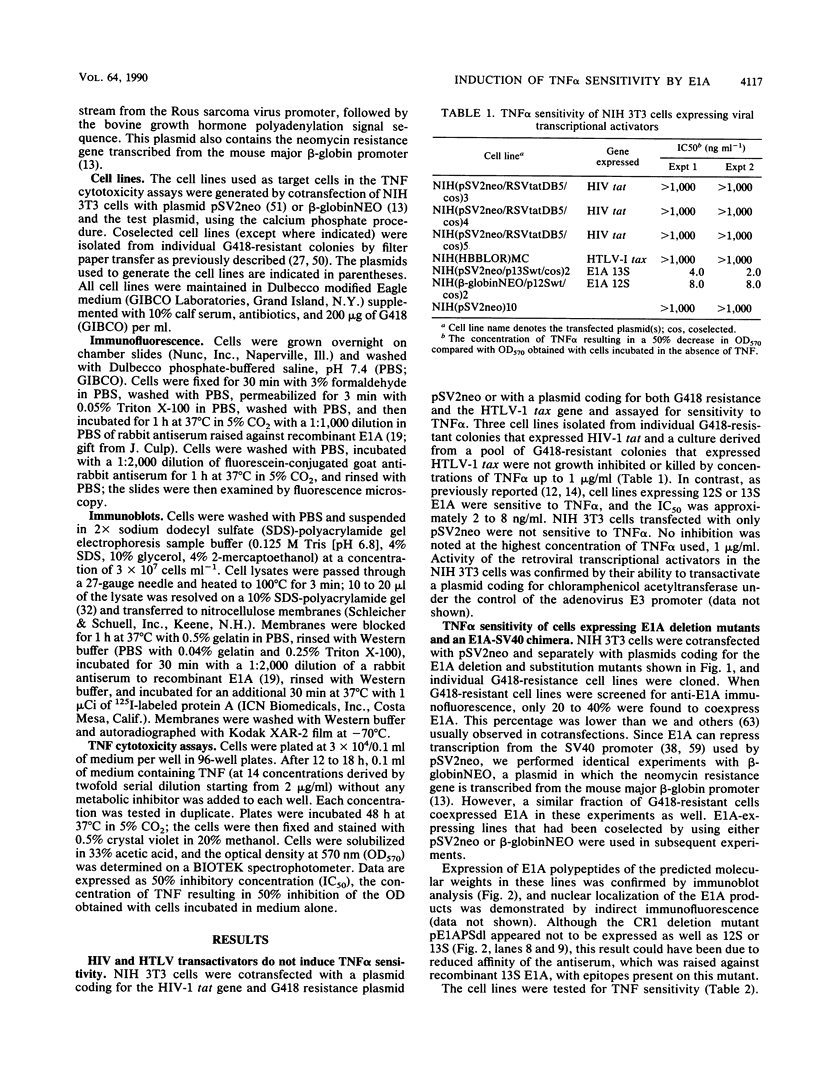
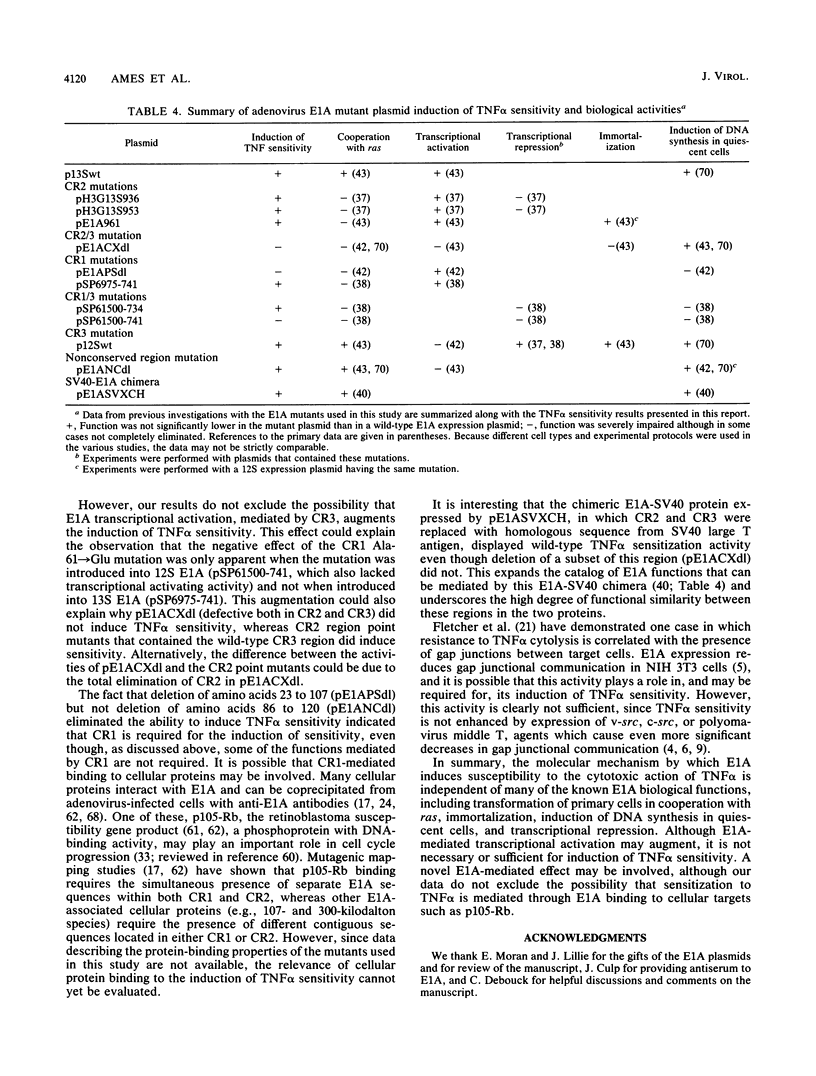
We have previously shown that expression of the adenovirus E1A 12S or 13S products in NIH 3T3 fibroblasts induces susceptibility to the cytotoxic actions of tumor necrosis factor alpha (TNF alpha). A large number of studies have mapped the multiple biological functions of the 12S and 13S products to three highly conserved regions (CR) within the E1A sequence. Here we used plasmids coding for E1A deletion and point mutants in these regions to generate target cell lines for TNF alpha cytotoxicity assays to determine which regions and functions are necessary for the induction of TNF alpha sensitivity. Expression of CR1 was required for the induction of TNF alpha sensitivity. This finding did not reflect a requirement for transforming or transcriptional repression activity, since some mutants that were defective in both of these properties were able to induce TNF alpha sensitivity. CR2 transformation-defective point mutants, but not a CR2/3 region deletion mutant, were also able to induce sensitivity. In addition, NIH 3T3 cells expressing the retroviral transcription activators tat from human immunodeficiency virus type 1 and tax from human T-lymphotropic virus type I were not sensitive to TNF alpha. However, the possibility that E1A-mediated transcriptional activation can augment the induction of TNF alpha sensitivity is not excluded. Comparison of data from previous biological studies with the TNF alpha cytotoxicity assays presented here suggested that the mechanism by which E1A induces sensitivity to TNF alpha in NIH 3T3 cells is independent of many of the known E1A biological functions, including transformation in cooperation with ras, immortalization, induction of DNA synthesis in quiescent cells, and transcriptional repression. A novel E1A-mediated effect may be involved, although our data do not exclude the possibility that sensitization to TNF alpha is mediated through E1A binding to cellular proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Yamamoto T., Iihara S., Yamazaki M., Mizuno D. A possible role of glucocorticoids: an intrinsic inhibitor of the cytotoxic activity of tumor necrosis factor. Jpn J Cancer Res. 1988 Mar;79(3):305–308. doi: 10.1111/j.1349-7006.1988.tb01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S., Drysdale B. E., Shin H. S. Tumor necrosis factor-mediated cytotoxicity involves ADP-ribosylation. J Immunol. 1988 Jun 15;140(12):4187–4192. [PubMed] [Google Scholar]
- Azarnia R., Loewenstein W. R. Polyomavirus middle T antigen downregulates junctional cell-to-cell communication. Mol Cell Biol. 1987 Feb;7(2):946–950. doi: 10.1128/mcb.7.2.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarnia R., Mitcho M., Shalloway D., Loewenstein W. R. Junctional intercellular communication is cooperatively inhibited by oncogenes in transformation. Oncogene. 1989 Oct;4(10):1161–1168. [PubMed] [Google Scholar]
- Azarnia R., Reddy S., Kmiecik T. E., Shalloway D., Loewenstein W. R. The cellular src gene product regulates junctional cell-to-cell communication. Science. 1988 Jan 22;239(4838):398–401. doi: 10.1126/science.2447651. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Trosko J. E., Kung H. J., Bombick D., Matsumura F. Potential role of the src gene product in inhibition of gap-junctional communication in NIH/3T3 cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5360–5364. doi: 10.1073/pnas.82.16.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapekar M. S., Glazer R. I. The synergistic cytocidal effect produced by immune interferon and tumor necrosis factor in HT-29 cells is associated with inhibition of rRNA processing and (2',5') oligo (A) activation of RNase L. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1180–1187. doi: 10.1016/s0006-291x(88)80490-x. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Cann A. J., Shah N. P., Gaynor R. B. Functional relation between HTLV-II x and adenovirus E1A proteins in transcriptional activation. Science. 1985 Nov 1;230(4725):570–573. doi: 10.1126/science.2996140. [DOI] [PubMed] [Google Scholar]
- Chen M. J., Holskin B., Strickler J., Gorniak J., Clark M. A., Johnson P. J., Mitcho M., Shalloway D. Induction by E1A oncogene expression of cellular susceptibility to lysis by TNF. Nature. 1987 Dec 10;330(6148):581–583. doi: 10.1038/330581a0. [DOI] [PubMed] [Google Scholar]
- Connors R. W., Sweet R. W., Noveral J. P., Pfarr D. S., Trill J. J., Shebuski R. J., Berkowitz B. A., Williams D., Franklin S., Reff M. E. DHFR coamplification of t-PA in DHFR+ bovine endothelial cells: in vitro characterization of the purified serine protease. DNA. 1988 Nov;7(9):651–661. doi: 10.1089/dna.1988.7.651. [DOI] [PubMed] [Google Scholar]
- Cook J. L., May D. L., Wilson B. A., Holskin B., Chen M. J., Shalloway D., Walker T. A. Role of tumor necrosis factor-alpha in E1A oncogene-induced susceptibility of neoplastic cells to lysis by natural killer cells and activated macrophages. J Immunol. 1989 Jun 15;142(12):4527–4534. [PubMed] [Google Scholar]
- Cook J. L., May D. L., Wilson B. A., Walker T. A. Differential induction of cytolytic susceptibility by E1A, myc, and ras oncogenes in immortalized cells. J Virol. 1989 Aug;63(8):3408–3415. doi: 10.1128/jvi.63.8.3408-3415.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerksen-Hughes P., Wold W. S., Gooding L. R. Adenovirus E1A renders infected cells sensitive to cytolysis by tumor necrosis factor. J Immunol. 1989 Dec 15;143(12):4193–4200. [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduchi E., Alonso M. A., Carrasco L. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol. 1989 Mar;63(3):1354–1359. doi: 10.1128/jvi.63.3.1354-1359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B., Krippl B., Andrisani O., Jones N., Westphal H., Rosenberg M. E1A 13S and 12S mRNA products made in Escherichia coli both function as nucleus-localized transcription activators but do not directly bind DNA. Mol Cell Biol. 1985 Oct;5(10):2653–2661. doi: 10.1128/mcb.5.10.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher W. H., Shiu W. W., Ishida T. A., Haviland D. L., Ware C. F. Resistance to the cytolytic action of lymphotoxin and tumor necrosis factor coincides with the presence of gap junctions uniting target cells. J Immunol. 1987 Aug 1;139(3):956–962. [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Elmore L. W., Tollefson A. E., Brady H. A., Wold W. S. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell. 1988 May 6;53(3):341–346. doi: 10.1016/0092-8674(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Green M., Loewenstein P. M., Pusztai R., Symington J. S. An adenovirus E1A protein domain activates transcription in vivo and in vitro in the absence of protein synthesis. Cell. 1988 Jun 17;53(6):921–926. doi: 10.1016/s0092-8674(88)90429-1. [DOI] [PubMed] [Google Scholar]
- Harlow E., Whyte P., Franza B. R., Jr, Schley C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol Cell Biol. 1986 May;6(5):1579–1589. doi: 10.1128/mcb.6.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Baba M., Sato A., Hirabayashi K., Tanabe F., Shigeta S., De Clercq E. Tumor necrosis factor enhances replication of human immunodeficiency virus (HIV) in vitro. Biochem Biophys Res Commun. 1989 Jan 16;158(1):307–312. doi: 10.1016/s0006-291x(89)80213-x. [DOI] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Mymryk J. S., Evelegh C. M., Cunniff N. F., Bayley S. T. Sequences in E1A proteins of human adenovirus 5 required for cell transformation, repression of a transcriptional enhancer, and induction of proliferating cell nuclear antigen. Virology. 1989 Jul;171(1):120–130. doi: 10.1016/0042-6822(89)90518-7. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., Coussens P. M., Danko A. V., Shalloway D. Overexpressed pp60c-src can induce focus formation without complete transformation of NIH 3T3 cells. Mol Cell Biol. 1985 May;5(5):1073–1083. doi: 10.1128/mcb.5.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelekar A., Cole M. D. Immortalization by c-myc, H-ras, and Ela oncogenes induces differential cellular gene expression and growth factor responses. Mol Cell Biol. 1987 Nov;7(11):3899–3907. doi: 10.1128/mcb.7.11.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Miller J. S., Porter D., Roberts B. E. E1a regions of the human adenoviruses and of the highly oncogenic simian adenovirus 7 are closely related. J Virol. 1985 Feb;53(2):399–409. doi: 10.1128/jvi.53.2.399-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff W. C., Fann A. V. Human tumor necrosis factor-alpha kills herpesvirus-infected but not normal cells. Lymphokine Res. 1986 Summer;5(3):215–221. [PubMed] [Google Scholar]
- Kuppuswamy M., Subramanian T., Chinnadurai G. Separation of immortalization and T24-ras oncogene cooperative functions of adenovirus E1a. Oncogene. 1988 Jun;2(6):613–615. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Cook J. L. A new role for DNA virus early proteins in viral carcinogenesis. Science. 1985 Jan 4;227(4682):15–20. doi: 10.1126/science.3843807. [DOI] [PubMed] [Google Scholar]
- Liddil J. D., Dorr R. T., Scuderi P. Association of lysosomal activity with sensitivity and resistance to tumor necrosis factor in murine L929 cells. Cancer Res. 1989 May 15;49(10):2722–2728. [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Transcription activation by the adenovirus E1a protein. Nature. 1989 Mar 2;338(6210):39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Moran B., Zerler B. Interactions between cell growth-regulating domains in the products of the adenovirus E1A oncogene. Mol Cell Biol. 1988 Apr;8(4):1756–1764. doi: 10.1128/mcb.8.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onclercq R., Gilardi P., Lavenu A., Cremisi C. c-myc products trans-activate the adenovirus E4 promoter in EC stem cells by using the same target sequence as E1A products. J Virol. 1988 Dec;62(12):4533–4537. doi: 10.1128/jvi.62.12.4533-4537.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr D. S., Rieser L. A., Woychik R. P., Rottman F. M., Rosenberg M., Reff M. E. Differential effects of polyadenylation regions on gene expression in mammalian cells. DNA. 1986 Apr;5(2):115–122. doi: 10.1089/dna.1986.5.115. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Miller J. S., Kimelman D., Cepko C. L., Lemischka I. R., Mulligan R. C. Individual adenovirus type 5 early region 1A gene products elicit distinct alterations of cellular morphology and gene expression. J Virol. 1985 Nov;56(2):404–413. doi: 10.1128/jvi.56.2.404-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routes J. M., Cook J. L. Adenovirus persistence in man. Defective E1A gene product targeting of infected cells for elimination by natural killer cells. J Immunol. 1989 Jun 1;142(11):4022–4026. [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Shalloway D., Johnson P. J., Freed E. O., Coulter D., Flood W. A., Jr Transformation of NIH 3T3 cells by cotransfection with c-src and nuclear oncogenes. Mol Cell Biol. 1987 Oct;7(10):3582–3590. doi: 10.1128/mcb.7.10.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Suffys P., Beyaert R., Van Roy F., Fiers W. Reduced tumour necrosis factor-induced cytotoxicity by inhibitors of the arachidonic acid metabolism. Biochem Biophys Res Commun. 1987 Dec 16;149(2):735–743. doi: 10.1016/0006-291x(87)90429-3. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Timmers H. T., De Wit D., Bos J. L., Van der Eb A. J. E1A products of adenoviruses reduce the expression of cellular proliferation-associated genes. Oncogene Res. 1988;3(1):67–76. [PubMed] [Google Scholar]
- Timmers H. T., van Dam H., Pronk G. J., Bos J. L., Van der Eb A. J. Adenovirus E1A represses transcription of the cellular JE gene. J Virol. 1989 Mar;63(3):1470–1473. doi: 10.1128/jvi.63.3.1470-1473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga O., Yaegashi T., Lowe J., Dobbs L., Padmanabhan R. Sequence analysis in the E1 region of adenovirus type 4 DNA. Virology. 1986 Dec;155(2):418–433. doi: 10.1016/0042-6822(86)90204-7. [DOI] [PubMed] [Google Scholar]
- Tschachler E., Robert-Guroff M., Gallo R. C., Reitz M. S., Jr Human T-lymphotropic virus I-infected T cells constitutively express lymphotoxin in vitro. Blood. 1989 Jan;73(1):194–201. [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The Rb gene and the negative regulation of cell growth. Blood. 1989 Aug 1;74(2):529–532. [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- Yamauchi N., Kuriyama H., Watanabe N., Neda H., Maeda M., Niitsu Y. Intracellular hydroxyl radical production induced by recombinant human tumor necrosis factor and its implication in the killing of tumor cells in vitro. Cancer Res. 1989 Apr 1;49(7):1671–1675. [PubMed] [Google Scholar]
- Yee S. P., Branton P. E. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology. 1985 Nov;147(1):142–153. doi: 10.1016/0042-6822(85)90234-x. [DOI] [PubMed] [Google Scholar]
- Young K. S., Weigel R., Hiebert S., Nevins J. R. Adenovirus E1A-mediated negative control of genes activated during F9 differentiation. Mol Cell Biol. 1989 Jul;9(7):3109–3113. doi: 10.1128/mcb.9.7.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerler B., Roberts R. J., Mathews M. B., Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. doi: 10.1128/mcb.7.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam H., Offringa R., Smits A. M., Bos J. L., Jones N. C., van der Eb A. J. The repression of the growth factor-inducible genes JE, c-myc and stromelysin by adenovirus E1A is mediated by conserved region 1. Oncogene. 1989 Oct;4(10):1207–1212. [PubMed] [Google Scholar]




