Abstract
Though many neuronal cell fate decisions result in reproducible outcomes, stochastic choices often lead to spatial randomization of cell subtypes. This is often the case in sensory systems where expression of a specific sensory receptor gene is selected randomly from a set of possible outcomes. Here, we describe recent findings elucidating the mechanisms controlling color photoreceptor subtypes in flies and olfactory receptor subtypes in worms and mice. Although well-known biological concepts such as lateral signaling and promoter selection play roles in these cases, fundamental questions concerning these choice mechanisms remain.
Introduction
Many developmental phenomena involve processes that lead to determinate outputs. Lineage information and signaling cues at specific developmental timepoints, though varied at certain levels, are integrated to yield robust, reproducible cell fate executions. For example, the worm species C. elegans has a nearly invariant body plan down to the identity and number of cells. It develops to the adult stage via a defined lineage pattern where each cell translates historical and signaling information from its ancestors and neighboring cells. Though the worm represents an extreme example, determinate cell fate induction during development is a general rule that insures the reproducible formation of an evolutionarily favored body plan and prevents wild variations. While most cell fate decisions are fixed within developmental contexts, not all are determinate. Some cell fate decisions involve random selection among alternatives based on variations within a cell or between cells. Similar to determinate decisions, stochastic cell fate decisions are maintained to yield robust, inconvertible outcomes. Some of the best-studied stochastic cell fate decisions occur in sensory neurons whose randomization of receptor expression generates diversification of function in otherwise identical cell types.
One of the best understood cases of stochastic cell fate choice involves selection of the M or L cone photoreceptor cell subtypes arranged randomly throughout the center of the human retina (fovea). The L and M opsin genes are located next to one another on the chromosome and are controlled by a common locus control region (LCR) of cis-regulatory DNA. In a given cone photoreceptor, the LCR loops to contact one of the two opsin promoters, thus activating expression from this promoter. The other opsin gene is not expressed since the LCR is not available to activate expression from its promoter. This mechanism allows for random, cell autonomous selection of cell fates leading to a stochastic distribution of the two types of cones within the retina[1].
In contrast with this cell autonomous choice, neuronal cell fate specification in the Drosophila central nervous system occurs through non-autonomous signaling and feedback mechanisms: One neuroblast is selected randomly from a field of epithelial cells, and this choice leads all of its neighbors to become epidermal cells. This process, called lateral inhibition, involves the classical Notch/Delta signaling system. Initially, all cells of the proneural cluster signal each other via Delta (ligand)-Notch (receptor) interactions. Due to subtle random fluctuations, one cell from the proneural cluster expresses slightly more Delta and signals to increase Notch activity in its neighbors. This increased Notch activity in the receiving cells leads to upregulation of Notch and downregulation of Delta, whereas the sending cell upregulates Delta and downregulates Notch. A feedback loop between several cells is established leading to high Delta expression and neuroblast fate in one cell and high Notch expression and epidermal fate in its neighbors. Since subtle variations between otherwise equivalent cells initiates this feedback mechanism, ablation of an eventual neuroblast cell will lead to replacement by another, unique cell in the cluster[2,3].
LCR activation and lateral signaling are two relatively well-understood mechanisms controlling stochastic decisions. This review will focus on recent findings regarding three stochastic neuronal fate decisions made in worms, flies, or mice. Though our understanding of the mechanisms controlling each cell fate decision is not yet completely clear, it is apparent that these systems seem to borrow conceptual paradigms from LCR-looping and lateral inhibition, but add their own wrinkles by utilizing vastly different molecular machinery or layering complexity through multiple tiers of decision making.
Selecting expression of one gene from 1300 possibilities
Perhaps the most complex example of stochastic neuronal subtype choice involves the mouse olfactory system. Nearly 5% of the mouse genome is dedicated to the ~1300 olfactory receptor genes (OR). From this battery of OR genes, each olfactory sensory neuron (OSN) selects to express one allele of one OR to the exclusion of all of the others. The process involves at least two mechanistic processes: selection of a single OR for expression and exclusion of all other ORs, including the second allele of the same gene. One early hypothesis centered on a recombination-based mechanism similar to immunoglobins in which mature OSNs would have permanent changes in their DNA. However, mice cloned from a differentiated OSN still display a full repertoire of OR expression effectively ruling this model out[4,5].
An alternative model focused on the involvement of specific regulatory DNA sequences. Serizawa and colleagues showed that the “H region”, a 2.1 kb region conserved between human and mouse, was required for the expression of ORs from a specific cluster. An exciting model was proposed by Lomvardos and coworkers who showed that the H region locus co-localizes with the actively transcribed OR gene, even if the genomic locus of this OR gene resides on another chromosome[6]. Further, adding copies of the H region leads to dysregulation of the exclusion machinery and co-expression of a functional OR gene with an OR pseudogene. The H region was thus proposed to function as a unique, global regulator of OR selection working as a trans-acting LCR. The H region would interact with the promoter of one OR gene to activate it and prevent any other locus (including the other allele) from being expressed[6]. However, two groups knocked out either the entire H region or a critical section and found that expression of only a neighboring cluster of OR genes was affected, with no global effect[7,8]. The H region thus plays an “LCR type role” in regulating local OR genes in cis but might not act as a global regulator.
Though seemingly ruling out the model of H region as a global regulator, these findings do raise additional questions. Why is the H region associated with actively transcribed OR genes if it does not regulate their activity? Perhaps, all OR promoters (controlling either a single gene or a gene cluster) are localized to a single position in the nucleus where some “selector” machinery activates expression of a single OR promoter. In this model, each OR gene would be controlled by an H-like regulatory region. These H-like regions would all be restricted to one specific nuclear location. At this nuclear locus, “selector” molecular machinery would activate in trans expression of only one H-like region leading to repression of all others (via a failure to activate) (Fig. 1a). In the case of the cluster regulated by the H region, there would be two levels of selection: first, selection of the H region enhancer, and then “LCR type” selection within the OR cluster whereby the H-region would itself select in cis one of the genes from the nearby cluster (Fig. 1b). Identification and characterization of an independent H-region like enhancer will be key to test such an idea. One excellent H-like candidate is the well-characterized promoter of the M71 OR locus. Both the M71 promoter and core H region contain homeodomain and O/E-like binding sites that are required for activity[9]. O/E proteins compose a family of at least four transcription factors thought to play a role in OSN-specific gene regulation[10,11]. It will be interesting to see if the M71 promoter is similarly localized to sites of active OR transcription. Furthermore, it will be important to test whether adding copies of the H regions that induce ectopic expression leads to the formation of one or several nuclear locations of active transcription. Along the same lines, DNA regions surrounding the deleted H region should be tested for localization. The H region may not actively regulate this OR cluster but rather bring the DNA region to the nuclear machinery for regulation. Thorough analyses of the localization of known cis-regulatory regions with actively transcribed or repressed OR genes would address whether recruitment to a specific nuclear locus is required for OR selection.
Fig. 1. Olfactory subtype selection in mice.
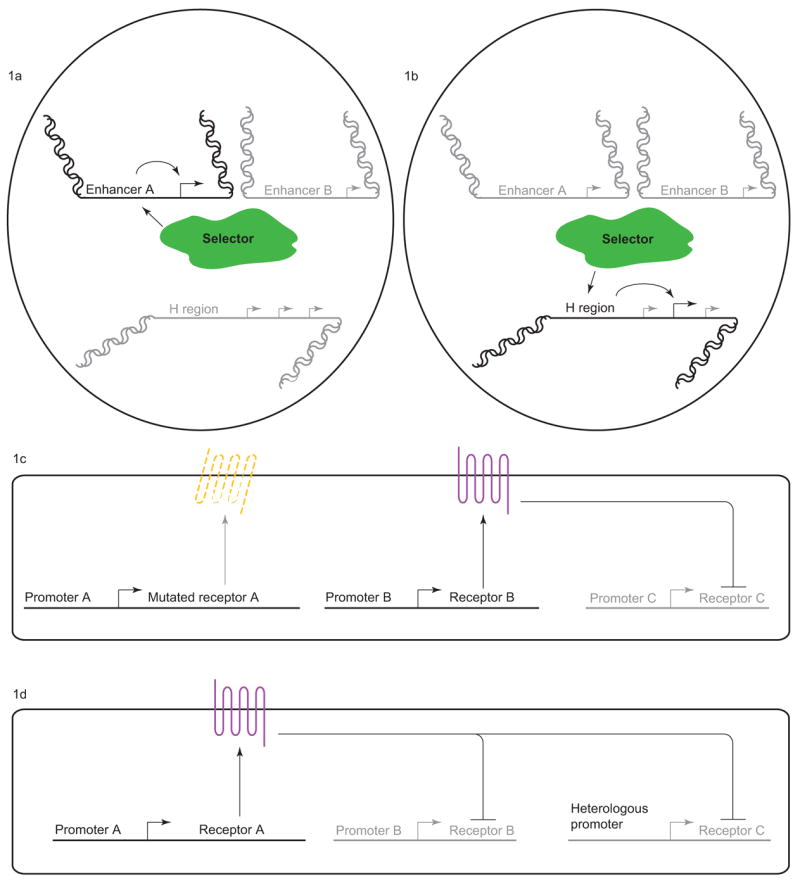
(a) “Selector” machinery activates expression of a single OR gene. (b) “Selector” machinery activates the H-region enhancer which selects an OR gene from a proximal cluster for expression. (c) OR protein coding region is required for repression of other OR genes. (d) OR protein coding regions contain cis-regulatory elements that mediate repression despite the presence of a heterologous promoter.
Once an OR is selected for expression, exclusion of all other ORs occurs to prevent sensory confusion from multiple inputs. In a critical observation, Serizawa and colleagues found that repression of other ORs in OSNs expressing a transgene carrying a specific OR locus was dependent on the presence of the coding region of the OR [12]. Deletion or frameshift of the OR coding region in the transgene leads to de-repression of another OR (Fig. 1c) [12]. Together, these observations suggest that a translated OR protein is required to establish single receptor choice. However, canonical olfactory signal transduction activity is not required since transgenic ORs that carry a mutation in the G-protein binding site are still capable of repression[13]. Interestingly, the OR DNA coding region itself likely contains cis-regulatory sites for repression since transcription of an OR under the control of a heterologous promoter is inhibited by the presence of another active locus (Fig. 1d). It seems that timing may be key to this regulation since early expression under a heterologous promoter allows expression of the transgenic OR, whereas late expression does not[13].
This description highlights the fact that, despite intense work in the field, the mechanisms controlling selection of a single OR gene allele and exclusion of all others remain mysterious. The main insights have come through characterization of cis-regulatory regions controlling OR gene expression. It seems that expanding such analyses in vivo and computationally to evaluate multiple OR promoters and enhancers will be essential. Identification of the transcription factors binding the homeodomain and O/E-like elements in the H region and the M71 promoter may lead to further elucidation of these control mechanisms. The LIM-homeodomain protein, Lhx2, binds the M71 promoter and has been characterized to be required for expression of all tested OR genes of one class (class II, including M71) while having nearly no effect on another class of OR genes (class I). However, Lhx2 is expressed in all OSNs and is therefore not sufficient for OR gene expression in a general way[14,15]. A critical mechanistic question is whether OR gene regulation by trans-acting factors occurs via restrictive expression or global expression with selective activity. In the next section, we discuss a stochastic fate choice involving color photoreceptor subtype selection in flies which is controlled by the restrictive expression of a specific transcriptional regulator.
Creating a color vision photoreceptor mosaic
The Drosophila eye has been utilized for decades as a model to analyze various biological processes, from cell cycle to cell fate determination. The eye is a hexagonal grid of ~800 ommatidia, or unit eyes. Each ommatidium contains eight photoreceptor cells, subdivided into two types: six outer photoreceptors (R1-6) whose main function is motion detection, and two inner photoreceptors, R7 and R8, which are involved in color discrimination. The inner photoreceptors sit at the center of the ommatidium with R7 and R8 pointing in the same direction. Ommatidia can be classified into two subtypes, pale or yellow, based on the expression of rhodopsin and the presence of pigments (Fig. 2a). In the yellow subtype, R7 contains the UV-sensitive Rhodopsin4 (Rh4) as well as a yellow filtering pigment while R8 expresses the green-sensitive Rh6. In the pale subtype, R7 contains a different UV-sensitive Rh3 while R8 expresses the blue-sensitive Rh5. These two subtypes are arranged randomly throughout the retina in a ratio of approximately 70% yellow to 30% pale.
Fig. 2. Color photoreceptor subtype selection in flies.
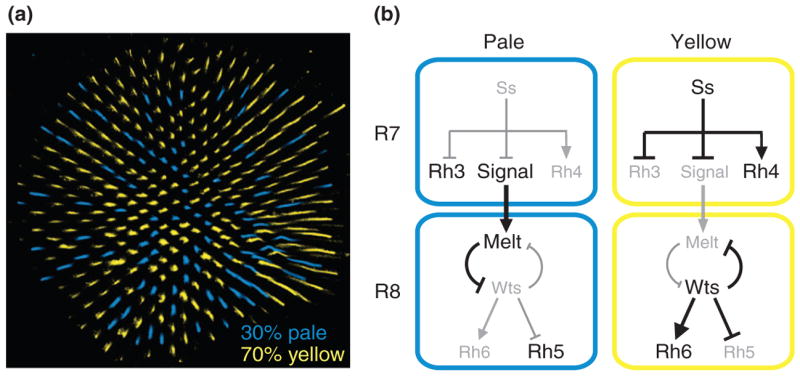
(a) The random mosaic of color photoreceptor expression. (b) Spineless controls the pale vs. yellow subtype choice.
This subtype decision appears to be determined in R7 and then instructed upon R8. In the absence of R8, R7 express either Rh3 or Rh4, suggesting that R7 can make the subtype decision independently. However, in the absence of R7s, R8s almost exclusively express Rh6 suggesting that induction of pale R8 fate (Rh5 expression) is dependent on a signal from R7[16]. These observations support a simple model in which subtype is decided in R7. R7s that choose pale fate then induce pale fate in their coordinate R8 partner. This signal from R7 to R8 controls a bistable loop of two key regulators: the tumor suppressor warts and the growth regulator melted. When a pale R7 cell provides a signal, the coordinate R8 cell upregulates melted and represses warts, leading to the expression of Rh5 and repression of Rh6. In the absence of the signal, warts is expressed, repressing melted and Rh5 expression and activating Rh6 expression [17].
R7 therefore makes a critical decision that not only controls its own subtype fate but also the fate of R8. How does R7 make this choice? The PAS-bHLH transcription factor, Spineless, is a key player. Spineless is expressed in a subset of R7s where it is necessary and sufficient to induce the yellow fate (Fig. 2b) [18]. Thus, the spineless gene appears to be an integrator of noise within the subtype decision machinery. The nature of this noise, either external, through the integration of multiple signals, or internal via random variations in transcriptional or post-transcriptional output, is not known. Interestingly, spineless mutants display a high level of induction of pale R8 fate (as would be expected if R7 is the subtype decision maker that instructs R8). However, this induction is not 100% suggesting that either spineless mutants exhibit a breakdown in the signaling mechanism, or that the coordination mechanism involves a more complicated interaction between R7 and R8. Similarly, loss of pale R8 fate induction in sevenless mutants is not complete (i.e. there is a small percentage of R8s that express Rh5). This suggests that R8 is competent to make an independent stochastic choice and that R7 to R8 communication may not only involve activation of one fate but also repression of the other. As our understanding of the system progresses, it will be interesting to see how the intrinsic decision making abilities are modified through external cues. In other words, does the system work via the simple model where R7 is the decision maker and instructor of R8 fate, or are two inherent stochastic cell fate decisions made in both R7 and in R8 that are heavily biased through complex interactions between these cells? In the next section, we discuss such a case involving an olfactory subtype decision in worms. In this case, two bilaterally identical cells are each biased towards one of two subtype fates. However, lateral signaling through a novel mechanism insures that only one of the two cells establishes this fate, whereas the other cell takes on the complementary fate.
A random cell fate choice with biased beginnings
Though the nervous system of C. elegans with about 300 neurons makes up nearly a third of the total cells of the organism, it is rather limited in terms of possible number of cell types and synaptic connections. Much of the nervous system is made up of morphologically bilaterally symmetrical pairs of neurons[19]. Despite this apparent mirror image, there are several cases of lateralization of gene expression that allow for functional diversification. Most lateralization examples are biased (non-random asymmetry) such that the left neuron expresses a specific gene battery compared to the right neuron. The AWC class of olfactory neurons is a unique case of antisymmetry (random asymmetry) in which expression of an olfactory receptor, str-2, occurs in either of the two neurons with approximately equal probability (str-2 expression = AWCon; no expression = AWCoff) (Fig. 3a). The functional consequence of this asymmetry is a segregation of odor detection capabilities to allow for better odor discrimination[20].
Fig. 3. Olfactory subtype selection in worms.
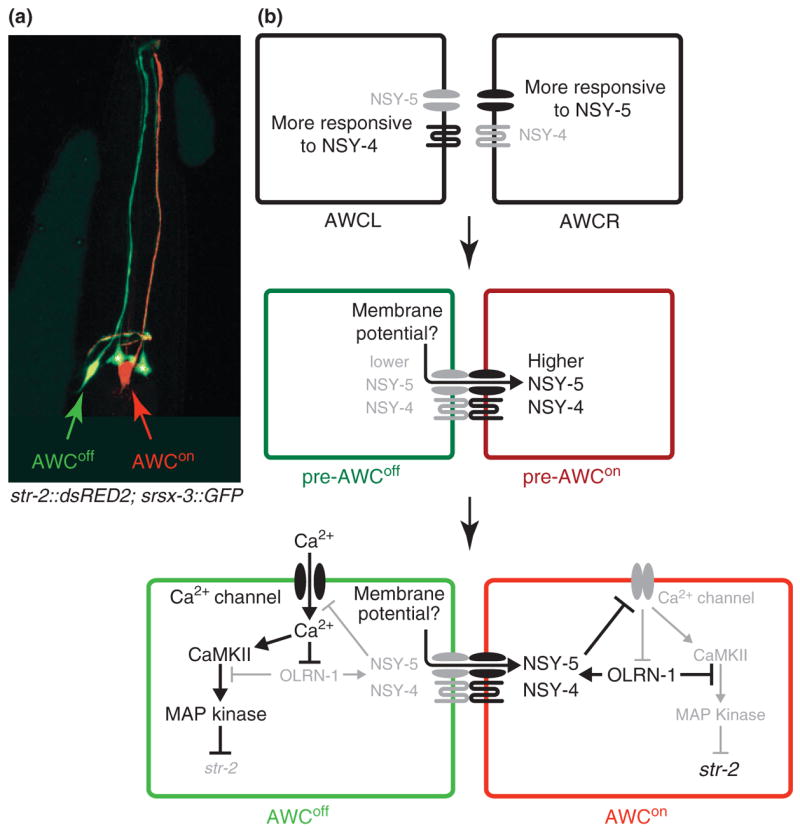
(a) Random expression of str-2 in AWC neurons. AWCon fate is determined by str-2 expression (in red). srsx-3 is a genetic marker for AWCoff fate (in green). Asterisks(*) indicate expression in non-AWC cells. (b) AWCL is biased to NSY-4 activity whereas AWCR is biased to NSY-5 activity. Lateral inhibition via a Ca2+ mediated mechanism promotes AWCon fate (high NSY-4 and NSY-5 activity) in a neuron while activating AWCoff fate (low NSY-4 and NSY-5 activity) in the other neuron.
In a series of elegant papers combining genetics, behavior analyses and electrophysiology, the Bargmann lab has identified a lateral inhibition mechanism controlling AWC asymmetry that is based on calcium signaling. They first showed that this asymmetry depends on communication between AWCL (left) and AWCR (right) since ablation of either neuron leads to the AWCoff state in the surviving neuron. Furthermore, mutants with axon guidance defects preventing contact between AWC neurons also leads to induction of the default AWCoff state in both AWCR and AWCL neurons. This interdependence suggests a lateral inhibition signaling mechanism whereby the molecular machinery inducing AWCon fate in one of the two AWC neurons inhibits the same fate in the other neuron. Yet Notch does not appear to play a role[21]. Rather, identification of the nsy-4 and nsy-5 genes revealed a mechanism involving Ca2+ signaling through a complex neuronal interaction network.
- nsy-4 encodes a transmembrane protein with similarity to a subunit of voltage-activated Ca2+ channels and the claudin multigene family of cell adhesion proteins. nsy-4 is required for the AWCon fate, and forced expression of NSY-4 in one AWC neuron always induces AWCon fate in that neuron and AWCoff fate in its wild type partner. This suggests that NSY-4 is not only required autonomously for the AWCon fate but is also involved in the non-autonomous signal to inhibit AWCon fate in the other AWC neuron[22].
- nsy-5 encodes an innexin-type gap junction protein that genetically acts in parallel with nsy-4. nsy-5 is required not only in AWC for the AWCon fate but also in several adjacent neurons to determine AWC asymmetry (i.e. to prevent the AWCon fate in the other AWC neuron). The adjacent neurons make a transient embryonic neural network linked by gap junctions that depends on nsy-5 activity[23].
NSY-4 and NSY-5 activity induces AWCon fate by repressing Ca2+ influx via voltage-gated calcium channels. In the AWC with lower NSY-4 and NSY-5 activity, Ca2+ influx triggers a kinase cascade in which Calmodulin KinaseII activates a downstream MAP kinase pathway to promote the AWCoff fate. In the AWC with higher NSY-4 and NSY-5 activity, inhibition of Ca2+ entry allows for de-repression of OLRN-1 activity, a novel negative regulator of MAP kinase signaling that reinforces the AWCon fate (Fig. 3b) [24–26].
Therefore, intercellular Ca2+ communication through a complex neural network is required for AWC asymmetry, yet the source of the initial variation between these neurons is not clear. Mosaic analyses of nsy-4 and nsy-5 provided a surprising observation that addresses the source and control of variation between these neurons. The response of AWCL and AWCR to NSY-4 and NSY-5 is differential such that AWCL responds to NSY-4 activity more efficiently than AWCR while NSY-5 activity is biased towards AWCR (Fig. 3b) [23]. Both AWC neurons appear to pursue AWCon fate through independent initial mechanisms, which apparently cancel one another at a roughly equivalent rate such that AWC asymmetry is anti-symmetric. Characterization of the mechanisms controlling this biased activity at the molecular and developmental level will be critical to identifying the source of variation determining this stochastic cell fate choice.
A separate example of lateral neuronal asymmetry in the worm may provide some clues into the developmental determination of biased AWC asymmetry. In the ASE pair of gustatory neurons, a biased asymmetry is determined by a Notch-mediated signaling event in the 4-cell embryo that is “remembered” through several cell divisions[27]. AWCL and AWCR share the same ancestor at the 4-cell stage but diverge at the subsequent cell division. Perhaps, a separate Notch or other signaling event creates diversity between these lineages at this time, which is later interpreted to generate biased response to NSY-4 and NSY-5 activity. An interesting question involves the evolution of these mechanisms: did stochastic choices arise from a biased asymmetry or did the biased asymmetry arise to make the stochastic choice more efficient? Analyses in related worm species, particularly a species that has lost (or never attained) this asymmetry, may provide some insight.
Though progress has been made in identifying the key molecular players involved in controlling AWC asymmetry, the actual mechanism in which variation between AWCL and R translates into robust cell fate choice still remains elusive. Ca2+ flow appears to play a critical role. Since electrophysiology in the worm embryo is a formidable task, future directions might focus on using Ca2+ sensitive fluorescent reporters to measure differences in Ca2+ levels amongst the members of the transient embryonic neural network during development. Measuring the differences in Ca+2 levels as the lateral signaling event occurs is only the first challenge. Finding the source of the variation will truly address the underlying mechanism controlling this cell fate decision. Measuring Ca2+ levels when members of the neural network have been removed by laser ablation may yield measurement of endogenous variations in Ca2+ influx. Since many of the key molecular players have been identified, the AWCL/R cell fate decision presents a great opportunity to address the source and control of variation between cells (or amongst members of a neural network).
Conclusion
Stochastic cell fate decisions are one of the most intriguing phenomena in biology. As scientists, we thrive on studying robust, reproducible outcomes; randomness is difficult to explain, making it all that more interesting. It appears that nature uses randomness to diversify neuronal subtype function, but how were such mechanisms set up during evolution? Are the roots of these decisions in biased subtype specification, which have adopted new mechanisms to diversify? Furthermore, it seems that the implementation of the use of stochastic cell fate decisions varies between organisms. For example, as described above, mice and worms randomly select olfactory receptors yet flies have a fixed pattern of receptor expression. In contrast to the random mosaic of photoreceptor expression in flies and humans, fish display a fixed lattice of expression.
Do the mechanisms found to regulate stochastic cell fate choices have roles in other contexts? For example, Notch signaling which is critical for lateral inhibition in neuroblast determination and other stochastic events also plays roles in determinate cell fate decisions such as the early embryonic lineage determination in worms and the specification of specific photoreceptor types in the fly eye. It will be interesting to see if Ca+2 mediated lateral inhibition or LCR-type cis regulation is used in a controlled and directed way to determine fixed outcomes in other biological contexts.
Finally, as our understanding of stochastic cell fate decisions becomes clearer, the next challenge will be determining the interpretation of spatially randomized information by downstream interneurons. OSNs that express the same OR project to the same glomerulus. However, these OSNs are randomly placed spatially: there must be a mechanism to coordinate the projections of OSNs that express the same OR gene. Perhaps even more perplexing is the interpretation of color information downstream of the color photoreception in the fly. The randomized subtypes of photoreceptors signal to an organized system of columnar and noncolumnar interneurons. Are there columnar interneuron subtypes that coordinate with photoreceptor subtype? If not, how does an organized lattice of neurons interpret spatially randomized information in a coherent way? An in-depth analysis of the more than 50 interneuron subtypes will be key[28].
Acknowledgments
We would like to thank Cori Bargmann, Stefan Fuss, and Daniel Vasiliauskas for helpful comments and discussion. Robert J. Johnston Jr. is supported by a Jane Coffin Childs Memorial Fund fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24:299–312. doi: 10.1016/s0896-6273(00)80845-4. [DOI] [PubMed] [Google Scholar]
- 2.Doe CQ, Skeath JB. Neurogenesis in the insect central nervous system. Curr Opin Neurobiol. 1996;6:18–24. doi: 10.1016/s0959-4388(96)80004-3. [DOI] [PubMed] [Google Scholar]
- 3.Campos-Ortega JA. Asymmetic division: dynastic intricacies of neuroblast division. Curr Biol. 1997;7:R726–728. doi: 10.1016/s0960-9822(06)00367-8. [DOI] [PubMed] [Google Scholar]
- 4.Eggan K, Baldwin K, Tackett M, Osborne J, Gogos J, Chess A, Axel R, Jaenisch R. Mice cloned from olfactory sensory neurons. Nature. 2004;428:44–49. doi: 10.1038/nature02375. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Ishii T, Feinstein P, Mombaerts P. Odorant receptor gene choice is reset by nuclear transfer from mouse olfactory sensory neurons. Nature. 2004;428:393–399. doi: 10.1038/nature02433. [DOI] [PubMed] [Google Scholar]
- 6.Lomvardas S, Barnea G, Pisapia DJ, Mendelsohn M, Kirkland J, Axel R. Interchromosomal interactions and olfactory receptor choice. Cell. 2006;126:403–413. doi: 10.1016/j.cell.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 7.Fuss SH, Omura M, Mombaerts P. Local and cis effects of the H element on expression of odorant receptor genes in mouse. Cell. 2007;130:373–384. doi: 10.1016/j.cell.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Nishizumi H, Kumasaka K, Inoue N, Nakashima A, Sakano H. Deletion of the core-H region in mice abolishes the expression of three proximal odorant receptor genes in cis. Proc Natl Acad Sci U S A. 2007;104:20067–20072. doi: 10.1073/pnas.0706544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothman A, Feinstein P, Hirota J, Mombaerts P. The promoter of the mouse odorant receptor gene M71. Mol Cell Neurosci. 2005;28:535–546. doi: 10.1016/j.mcn.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Wang SS, Tsai RY, Reed RR. The characterization of the Olf-1/EBF-like HLH transcription factor family: implications in olfactory gene regulation and neuronal development. J Neurosci. 1997;17:4149–4158. doi: 10.1523/JNEUROSCI.17-11-04149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SS, Betz AG, Reed RR. Cloning of a novel Olf-1/EBF-like gene, O/E-4, by degenerate oligo-based direct selection. Mol Cell Neurosci. 2002;20:404–414. doi: 10.1006/mcne.2002.1138. [DOI] [PubMed] [Google Scholar]
- 12.Serizawa S, Miyamichi K, Nakatani H, Suzuki M, Saito M, Yoshihara Y, Sakano H. Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science. 2003;302:2088–2094. doi: 10.1126/science.1089122. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen MQ, Zhou Z, Marks CA, Ryba NJ, Belluscio L. Prominent roles for odorant receptor coding sequences in allelic exclusion. Cell. 2007;131:1009–1017. doi: 10.1016/j.cell.2007.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirota J, Omura M, Mombaerts P. Differential impact of Lhx2 deficiency on expression of class I and class II odorant receptor genes in mouse. Mol Cell Neurosci. 2007;34:679–688. doi: 10.1016/j.mcn.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Hirota J, Mombaerts P. The LIM-homeodomain protein Lhx2 is required for complete development of mouse olfactory sensory neurons. Proc Natl Acad Sci U S A. 2004;101:8751–8755. doi: 10.1073/pnas.0400940101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou WH, Huber A, Bentrop J, Schulz S, Schwab K, Chadwell LV, Paulsen R, Britt SG. Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development. 1999;126:607–616. doi: 10.1242/dev.126.4.607. [DOI] [PubMed] [Google Scholar]
- 17.Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, Cohen S, Desplan C. The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell. 2005;122:775–787. doi: 10.1016/j.cell.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobert O, Johnston RJ, Jr, Chang S. Left-right asymmetry in the nervous system: the Caenorhabditis elegans model. Nat Rev Neurosci. 2002;3:629–640. doi: 10.1038/nrn897. [DOI] [PubMed] [Google Scholar]
- 20.Wes PD, Bargmann CI. C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature. 2001;410:698–701. doi: 10.1038/35070581. [DOI] [PubMed] [Google Scholar]
- 21.Troemel ER, Sagasti A, Bargmann CI. Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell. 1999;99:387–398. doi: 10.1016/s0092-8674(00)81525-1. [DOI] [PubMed] [Google Scholar]
- 22.Vanhoven MK, Bauer Huang SL, Albin SD, Bargmann CI. The claudin superfamily protein nsy-4 biases lateral signaling to generate left-right asymmetry in C. elegans olfactory neurons. Neuron. 2006;51:291–302. doi: 10.1016/j.neuron.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Chuang CF, Vanhoven MK, Fetter RD, Verselis VK, Bargmann CI. An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell. 2007;129:787–799. doi: 10.1016/j.cell.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 24.Bauer Huang SL, Saheki Y, Vanhoven MK, Torayama I, Ishihara T, Katsura I, van der Linden A, Sengupta P, Bargmann CI. Left-right olfactory asymmetry results from antagonistic functions of voltage-activated calcium channels and the Raw repeat protein OLRN-1 in C. elegans. Neural Develop. 2007;2:24. doi: 10.1186/1749-8104-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka-Hino M, Sagasti A, Hisamoto N, Kawasaki M, Nakano S, Ninomiya-Tsuji J, Bargmann CI, Matsumoto K. SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in Caenorhabditis elegans. EMBO Rep. 2002;3:56–62. doi: 10.1093/embo-reports/kvf001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sagasti A, Hisamoto N, Hyodo J, Tanaka-Hino M, Matsumoto K, Bargmann CI. The CaMKII UNC-43 activates the MAPKKK NSY-1 to execute a lateral signaling decision required for asymmetric olfactory neuron fates. Cell. 2001;105:221–232. doi: 10.1016/s0092-8674(01)00313-0. [DOI] [PubMed] [Google Scholar]
- 27.Poole RJ, Hobert O. Early embryonic programming of neuronal left/right asymmetry in C. elegans. Curr Biol. 2006;16:2279–2292. doi: 10.1016/j.cub.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 28.Morante J, Desplan C. in press. [Google Scholar]


