Abstract
| Strategy, Management and Health Policy
| ||||
| Enabling Technology, Genomics, Proteomics | Preclinical Research | Preclinical Development Toxicology, Formulation Drug Delivery, Pharmacokinetics | Clinical Development Phases I–III Regulatory, Quality, Manufacturing | Postmarketing Phase IV |
Individuals who suffer migraine, particularly migraine with visual aura (MwA), are susceptible to physiologically strong visual stimuli and find them aversive. Strong stimuli including bright light and certain visual patterns produce discomfort and perceptual illusions and can trigger migraine attacks. Perceptual illusions and visual discomfort are reported by most migraine sufferers and those with frequent headaches. These phenomena suggest that visual stimulation and consequent visual cortical activity contribute to the triggering of some migraine attacks. Perceptual illusions in MwA patients were found to be associated with hyper-activation in visual cortex. This excessive cortical activity is called visual stress. The cortex is generally hypothesized to be hyperexcitable in migraine, and this hyperexcitability could be the underlying mechanism of visual stress. It is hypothesized that visual stress results from too great a neural (hyperneural) activity in response to strong physiological sensory stimulation, particularly, but not exclusively, visual. A strong physiological visual input may cause a spread of excitation through hyperexcitable cortex, leading to neurons firing inappropriately and thereby resulting in perceptual illusions and distortions, and possibly promoting a migraine attack. Over the last 10 years, the use of colored filters to treat perceptual distortion of text has become common in many schools in Britain. The efficacy of precision spectral filters (PSF) in preventing migraine headache has been reported in several studies. One preliminary study revealed the suppressing effect of the PSF on visual cortical activity in a MwA patient, suggesting that it might be this reduction in cortical activation that is responsible for the reduction of the frequency of migraine attacks in those who benefited from the PSF. PSF offer a possible new prophylactic therapy for migraine. They are safe, free of side effects, and inexpensive.
Keywords: migraine, migraine with visual aura, visual stress, cortical hyperexcitability, precision spectral filters
INTRODUCTION
Migraine is a common neurovascular disorder, affecting approximately 28 million Americans with a high preponderance in women and high economic cost [Gerth et al., 2001; Hu et al., 1999]. Migraine headache pain can last hours to days. It is frequently accompanied by various visual disturbances. Photophobia and vision blurring are associated with most migraine attacks with or without aura. In patients experiencing aura, the aura is visual in 90% of cases. Bright light and certain visual patterns can trigger a migraine attack [Hay et al., 1994; Cao et al., 1999, 2002]. Perceptual illusions and visual discomfort are reported by most migraine sufferers and those with frequent headache [Wilkins, 1995; Marcus and Soso, 1989; Wilkins et al., 1984]. These phenomena suggest that visual stimulation and consequent activity in the visual cortex are factors in triggering some migraine attacks.
A general hypothesis in the pathophysiology of migraine is the hyperexcitability of the cerebrum [Welch et al., 1990]. One recent study supports the hypothesis that high susceptibility to perceptual illusions in the patients of migraine with visual aura (MwA) is associated with hyper-activation in visual cortex [Huang et al., 2003]. This excessive cortical activity is called visual cortical stress. Visual cortical stress, although produced by an experimental stimulus in the above study, can also result from such activities as reading, working on a computer, watching television, and seeing certain patterns in the day to day.
Over the last 10 years, the use of colored filters to treat perceptual distortion of text has become common in many schools in Britain [Jeanes et al., 1997; Wilkins and Lewis, 1999; Wilkins et al., 1996, 2001; Tyrrell et al., 1995]. The efficacy of precision spectral filters (PSF) in preventing headache has been reported in several studies [Wilkins, 1995; Wilkins et al., 1994, 2002]. One preliminary study revealed the suppressing effect of the PSF on visual cortical activity in a MwA patient, suggesting that it might be this reduction in cortical activation that is responsible for the reduction of the frequency of migraine attacks in those who benefited from the PSF [Huang et al., 2004; Wilkins et al., 2004]. In this report, we review relevant literature related to visual stress, migraine, and prophylaxis with PSF.
HYPEREXCITABILITY IN MIGRAINE
There are several disparate but convergent lines of evidence, recently reviewed [Welch, 2003], consistent with the hypothesis that in migraine the visual cortex is hyperexcitable: (1) four anticonvulsant drugs prevent migraine in randomized controlled trials [Krymchantowski et al., 2002]; (2) migraine and epilepsy are co-morbid conditions [Lipton et al., 1994]; (3) in migraineurs, transcortical magnetic stimulation of the visual cortex stimulates phosphenes more readily than in controls [Aurora and Welch, 1998]; (4) the evoked potential in migraineurs usually has a high amplitude and fails to show the expected habituation with repeated stimulation [Kropp and Gerber, 1995; Schoenen, 1996]; (5) DC-magneto-encephalography during visual stimulation reveals large-amplitude signals, reduced by the anticonvulsant, sodium valproate [Bowyer et al., 2005]. This cortical hyperexcitability could be caused by overactivity of the excitatory amino acids, glutamate and possibly aspartate, and imbalance of inhibitory neurotransmitters such as gamma-aminobutyric acid (GABA).
VISUAL RESPONSES IN MIGRAINE
Certain visual patterns are aversive to migraineurs [Marcus and Soso, 1989] and induce perceptual distortions to which migraineurs are particularly susceptible. These patterns induce seizures in patients with photosensitive epilepsy, and the susceptibility of migraineurs to such patterns is consistent with the above evidence of cortical hyperexcitability in migraine. Recently in a functional magnetic resonance imaging (fMRI) study, Huang et al. [2003] have shown that these patterns evoke an abnormally large blood oxygenation level dependent (BOLD) signal change in migraineurs. Figure 1 is taken from Huang et al. [2003] and shows the mean (±SE) changes in 6 controls and 6 MwA patients.
Fig. 1.
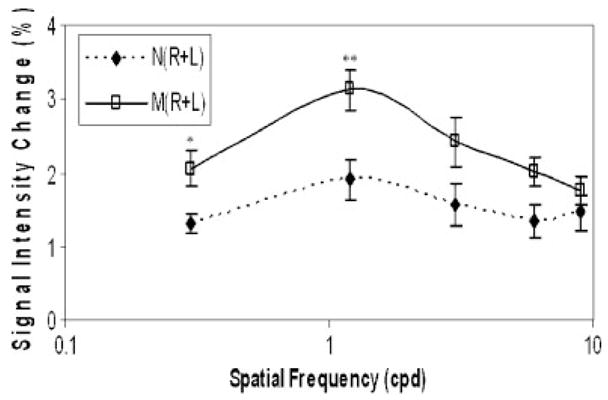
BOLD responses to black-white gratings with a spatial frequency of 0.3 to 9 cycles per degree (cpd) in MwA patients (M) and non-headache controls (N). *P<0.02; **P<0.005. (Reprinted from Huang et al., 2003, with permission from Wiley-Blackwell.)
STRONG PATTERNS
The grating patterns that induce the abnormal BOLD response in migraineurs also induce aversion [Marcus and Soso, 1989]. They are physiologically strong visual stimuli in the sense that (1) they have parameters optimal for visibility [Campbell and Robson, 1968]; (2) in studies of visual masking, they interfere maximally with the visibility of other stimuli with which they are combined [Chronicle and Wilkins, 1996]; (3) they induce high-amplitude visual-evoked potentials [Plant et al., 1983]; (4) they are associated with the greatest fMRI BOLD signal [Huang et al., 2003]; and (5) they induce seizures in patients with photosensitive epilepsy [Wilkins et al., 1979]. The patterns are those that might be expected to excite individual neurons within a cortical column most strongly.
VISUAL STIMULATION IN THE ENVIRONMENT
As has been seen, simple images, particularly patterns of stripes, are strong stimuli capable of provoking discomfort, headaches, and even seizures in susceptible persons. The patterns are aversive and epileptogenic provided the spatial frequency is within two octaves of 3 cycles per degree (cpd) [Wilkins et al., 1980, 1984).
More complex images, including those from art, from natural scenes, and from filtered noise can also evoke a sensation of discomfort [Fernandez and Wilkins, 2007]. The discomfort from such complex images can be predicted from the energy at different spatial scales in the image, as measured by the Fourier amplitude spectrum of the luminance. Whereas comfortable images show the regression of Fourier amplitude against spatial frequency common in natural scenes, uncomfortable images show a regression with disproportionately greater amplitude at spatial frequencies within two octaves of 3 cpd. This suggests that complex everyday images, as well as simple geometric patterns, may provoke discomfort via similar neurological mechanisms.
TRIGGERING OF HEADACHES
When asked, about 40% of patients with migraine will report visually provoked attacks [Hay et al., 1994]. A substantial proportion report that flicker induces attacks, and flicker is known to be epileptogenic. These reports are consistent with double-masked studies that have shown the imperceptible high-frequency flicker from fluorescent lighting [Wilkins et al., 1989] and computer screens [Kowacs et al., 2004] to be responsible for many headaches experienced by office workers. Some migraineurs are aware that stripes (such as those on clothing) can be a problem, and given the evidence in the previous paragraph, many environmental stimuli are a potential hazard in this way.
TEXT AS STRIPES
Text can be thought of as a striped pattern. The lines provide an image of horizontal stripes with a spatial frequency within two octaves of 3 cpd [Wilkins et al., 2004]. Individual words can also form a striped pattern. Words such as will and mum are striped because of the similarity of the neighboring letter strokes. The stripes within words (which are also within the aversive spatial frequency range) can be measured using the horizontal autocorrelation of the image of the word. “Striped” words, measured in this way, take longer to read, even for fluent readers. Reducing the periodicity of the stripes by varying the inter-stroke spacing can increase reading speed in poor readers [Wilkins et al., 2007].
PERCEPTUAL DISTORTIONS IN TEXT
Some people see distortions not only in stripes but also in text [Irlen, 1991] and it would appear that the stripes within text are responsible for the distortions [Wilkins and Nimmo-Smith, 1987; Wilkins et al., 2007]. It is now widely recognised that colored filters can reduce the distortions seen in text, and increase reading speed [Wilkins et al., 2001, 2005a; Wilkins, 2002; Wilkins and Lewis, 1999].
INDIVIDUAL DIFFERENCES
Individuals who see more distortions in striped patterns are those who read more quickly with their chosen colored filter [Hollis and Allen, 2006]. There is no one color that helps everyone. For reasons that are hard to explain, the best color needs to be individually selected and with precision [Wilkins et al., 2005a].
People who are susceptive to distortions can be identified objectively by the decrease in the speed of visual search that occurs when the search task is surrounded by an aversive pattern of stripes [Singleton and Henderson, 2007].
OPHTHALMIC TINTS (PSF) AND PERCEPTUAL DISTORTIONS
New ophthalmic tinting techniques are now in widespread use in Britain and elsewhere [Wilkins, 2003]. Over 25,000 tints have been prescribed in general optometric practice using the Intuitive Colorimeter [Wilkins and Sihra, 2000], a colorizer that illuminates text or patterns with colored light. In many individuals, it is possible to find a hue and saturation that reduces perceptual distortion and increases visual clarity and comfort. The appropriate color differs from one individual to another and has to be selected with precision for optimal effect [Wilkins et al., 2005b].
The spectral power distribution in the Intuitive Colorimeter is almost identical to that when the lenses are worn under conventional fluorescent lighting (CIE Type F3). The spectral match allows observers with color vision anomalies to be tested. The Intuitive Colorimeter system will provide lenses that closely approximate any chromaticity. The lenses have a smoothly varying spectral transmission that minimises metamerism [Wilkins, 2003].
Each individual reads most quickly under light of a particular individual optimal chromaticity and light that differs from the optimum in respect to hue or saturation results in slower reading. The greater the difference in color between the optimal color and that used for reading, the lower the reading speed, although when the CIE color difference (ΔE*) exceeds about 100, the speed is similar to that under white light [Wilkins et al., 2005a). (A color difference of 100 is similar to the difference in color between daylight and incandescent light). Despite this specificity, most tints (with the exception of purple) will provide at least some benefit under most types of lighting [Wilkins et al., 2005a).
BENEFIT IN VARIOUS NEUROLOGICAL DISORDERS
Precision spectral filters have now been shown to offer relief from visual symptoms in a variety of central nervous system disorders that involve the visual system. The disorders include photosensitive epilepsy [Wilkins et al., 1999], head injury [Padula and Shapiro, 1993], autism [Ludlow et al., 2006], multiple sclerosis [Newman Wright et al., 2007], and migraine [Wilkins et al., 2002]. In the migraine study, the effectiveness of PSF in the prevention of symptoms in the migraine sufferers was compared using a double-masked randomized controlled study with cross-over design. Seventeen patients chose the color of light that optimally reduced perceptual distortion of text and maximized clarity and comfort. A re-analysis of the headache diaries showed that in 45% of the MwA patients, the frequency of migraine headaches was reduced 50% or more during the days in which the PSF were worn compared to the days in which the control filters were worn. Although the sample size was small and the results suggestive rather than conclusive, the findings are similar to those of a previous double-masked study with children, which also showed a reduction of headaches with PSF [Wilkins at al., 1994].
All the above disorders are generally associated with an increased risk of seizures, consistent with the hypothesis that the filters are beneficial because of an elevated cortical hyperexcitability.
AN HYPOTHESIS
Strong patterns have spatial energy within a limited range of spatial frequency and orientation. They might, therefore, be expected to cause nearby columns of pyramidal neurons to fire within the visual cortex. Stellate interneurons provide inhibitory interconnections between pyramidal neurons and their inhibitory mechanisms are shared between these neurons. When there is strong excitation of neighboring pyramidal neurons, as, for example, when columns with similar orientation tuning are stimulated, inhibition may be insufficient to meet local demand, resulting in a spread of excitation. The spread may cause visual neurons to fire inappropriately and give rise to perceptual distortions. According to this hypothesis, an individual’s degree of susceptibility to distortions increases with, and reflects, the degree of cortical hyperexcitability. This is consistent with the increase in perceptual distortions observed after sleep deprivation [Wilkins et al., 1984].
Recent development in the understanding of color processing in the visual cortex reveals that it is not limited to discrete visual cortical area(s) (color centre(s)). Many cells in V1 respond to both equiluminant color and luminance modulation [Engel et al., 1997; Johnson et al., 2001]. Cells in V2 exhibit both color and disparity selectivity [Ts’o et al., 2001], while cells in V3 and V5 show wide variations in color-wavelength sensitivity [Zeki, 1990]. Gratings of different colors produce activation that peaks at different locations in macaque visual area V2, and the spatial organization across the cortical surface of the peak responses to the different colors is on the same order as color stimuli arranged on the CIE Uniform Chromaticity Scale (UCS) diagram [Xiao et al., 2003]. These data indicate that color and spatial processing interact and are intermingled in many visual areas. We can, therefore, expect that spectral filters will redistribute the cortical excitation that occurs in response to a visual stimulus.
It is hypothesized that in migraine the cortical hyperexcitability is non-uniform (non-uniformity is typical in other disorders such as epilepsy [Wilkins, 1995]. We hypothesize that “comfortable” colors redistribute the excitation in response to visual stimulation in such a way as to reduce the excitation in hyperexcitable regions.
A mechanism of this kind may explain the decrease in seizure susceptibility noted in a recent open trial of PSF in patients with photosensitive epilepsy [Wilkins et al., 1999]. It may also explain the therapeutic response to PSF in the above range of neurological disorders, and also the reduction in perceptual distortions, if these distortions are indeed due to a spread of excitation, as hypothesized above.
Functional imaging is currently being used to examine the hypothesis via investigating the effects of PSF on cortical activation in visual areas. In preliminary studies, a reduction of the fMRI BOLD abnormality using PSF has been demonstrated [Huang et al., 2004; Wilkins et al., 2004]. Figure 2 shows the suppressing effect of the PSF on visual cortical activity in a MwA patient. BOLD signal intensity responses in V1 and V2 were not different when the subject wore the PSF or control filters, and the maximum response occurred to 1.2 cpd, which was the same to the one shown in Figure 1. However, in V3, BOLD responses were reduced approximately 40% at a spatial frequency of 1.2 and 3.0 cpd when the subject wore the prescribed filters compared to the control gray or colored filters, showing that color can influence spatial processing and neural activation.
Fig. 2.
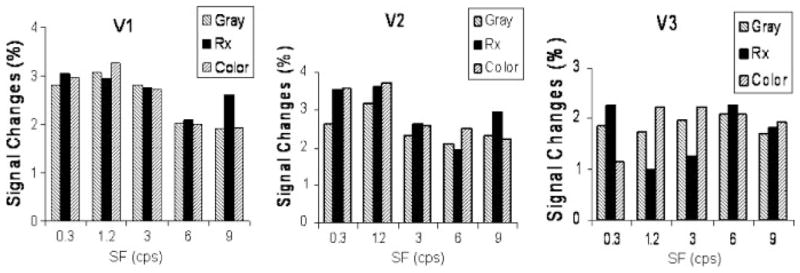
Grating-induced BOLD signal intensity changes in V1, V2, and V3 when the subject wore gray, prescribed, and control colored filters [Reprinted from Wilkins et al., 2004, which was published in the Journal of Research in Reading, a journal of the United Kingdom Literacy Association.] The activation is shown as a function of the spatial frequency (SF) of the grating (cpd).
A LINK WITH MIGRAINE AURA
As has already been described, individuals with migraine are unusually susceptible to the distortions seen in grating patterns. Their susceptibility increases in the 24 h prior to headache onset [Nulty et al., 1987] and, between headaches, susceptibility is greatest in the visual field affected by the aura. Grating patterns have been reported as triggering migraine aura in some patients. The aura is usually attributed to the cortical spreading depression (CSD) [Leao, 1944]. Cao et al. [1999] observed slowly spreading suppression of initial stimulus-evoked neuronal activity associated with hyper-oxygenation and blood volume increase in the occipital cortex during the early minutes of attacks of visually triggered MwA. In rabbits with a hyperexcitable cortex due to pre-treatment with metrazol, it is possible for sensory stimuli to provoke spreading depression [Van Harreveld and Stamm, 1955]. Furthermore, one recent study elicited the source of aura-related BOLD signal changes located in extrastriate visual cortex (V3A) rather than in V1 in one patient, the location of the source consistent with the type of patient’s typical aura [Hadjikhani et al., 2001]. Interestingly, in our preliminary study the PSF suppressed the cortical activation in V3 rather than in V1 and V2 in a MwA patient (Fig. 2).
SUMMARY
Visual stress is a factor in triggering some attacks in patients with migraine, particularly migraine with aura. Visual cortical hyperexcitability is considered to be responsible for visual stress. It is hypothesized that visual stress results from too great a neural activity in response to strong physiological sensory stimulation, a proposal that is supported by several recent neuroimaging studies. Precision spectral filters were found to be beneficial in reducing migraine headaches in a few migraine patients, particularly MwA patients. PSF offer a possible new prophylactic therapy for migraine. They are safe, free of side effects, and inexpensive.
Acknowledgments
National Institute of Neurological Disorders and Stroke; Grant number: R21NS054202.
References
- Aurora SK, Welch KM. Brain excitability in migraine: evidence from transcranial magnetic stimulation studies. Curr Opin Neurol. 1998;113:205–209. doi: 10.1097/00019052-199806000-00003. [DOI] [PubMed] [Google Scholar]
- Bowyer S, Mason KM, Moran JE, Tepley N, Mitsias PD. Cortical hyperexcitability in migraine patients before and after sodium valproate treatment. J Clin Neurophysiol. 2005;22:65–67. doi: 10.1097/01.wnp.0000150928.23523.a9. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Robson JG. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968;197:551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Welch KMA, Aurora S, Vikingstad EM. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol. 1999;56:548–554. doi: 10.1001/archneur.56.5.548. [DOI] [PubMed] [Google Scholar]
- Cao Y, Aurora SK, Nagesh V, Patel SC, Welch KM. Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology. 2002;59:72–78. doi: 10.1212/wnl.59.1.72. [DOI] [PubMed] [Google Scholar]
- Chronicle EP, Wilkins AJ. Gratings that induce distortions mask superimposed targets. Perception. 1996;25:661–668. doi: 10.1068/p250661. [DOI] [PubMed] [Google Scholar]
- Engel S, Zhang X, Wandell B. Colour tuning in human visual cortex measured with functional magnetic resonance imaging. Nature. 1997;388:68–71. doi: 10.1038/40398. [DOI] [PubMed] [Google Scholar]
- Fernandez D, Wilkins AJ. Uncomfortable images in art and nature. Perception. 2007 doi: 10.1068/p5814. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth WC, Carides GW, Dasbach EJ, Visser WH, Santanello NC. The multinational impact of migraine symptoms on healthcare utilisation and work loss. Pharmacoeconomics. 2001;19:197–206. doi: 10.2165/00019053-200119020-00006. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Sanchesez del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci. 2001;98:4687–4692. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay KM, Mortimer MJ, Barker DC, Debney LM, Good PA. 1044 women with migraine: the effect of environmental stimuli. Headache. 1994;34:166–168. doi: 10.1111/j.1526-4610.1994.hed3403166.x. [DOI] [PubMed] [Google Scholar]
- Hollis J, Allen PM. Screening for Meares-Irlen sensitivity in adults: can assessment methods predict changes in reading speed? Ophthal Physiol Opt. 2006;26:566–571. doi: 10.1111/j.1475-1313.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Arch Int Med. 1999;159:813–818. doi: 10.1001/archinte.159.8.813. [DOI] [PubMed] [Google Scholar]
- Huang J, Cooper TG, Satana D, Kaufman DI, Cao Y. Visual distortion provoked by a stimulus in migraine associated with hyperneuronal activity. Headache. 2003;43:664–671. doi: 10.1046/j.1526-4610.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- Huang J, Wilkins AJ, Cao Y. Mechanisms whereby precision spectral filters reduce visual stress: an fMRI study. Tenth Annual Meeting of the Organisation for Human Brain Mapping; Budapest, Hungary. June 13–17, 2004.2004. [Google Scholar]
- Irlen H. Reading by the colors: overcoming dyslexia and other reading disabilities through the Irlen method. New York: Avery Publishing Group; 1991. [Google Scholar]
- Jeanes R, Busby A, Martin J, Lewis E, Stevenson N, Pointon D, Wilkins AJ. Prolonged use of coloured overlays for classroom reading. Br J Psychol. 1997;88:531–548. [PubMed] [Google Scholar]
- Johnson EN, Hawken MJ, Shapley R. The spatial transformation of color in the primary visual cortex of the macaque monkey. Nature Neurosci. 2001;4:309–416. doi: 10.1038/86061. [DOI] [PubMed] [Google Scholar]
- Kowacs PA, Piovesan EJ, Werneck LC, Fameli H, Pereira da Silva H. Headache related to a specific screen flickering frequency band. Cephalalgia. 2004;24:408–410. doi: 10.1111/j.1468-2982.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- Kropp P, Gerber WD. Contingent negative variation during migraine attack and interval: evidence for nomalization of slow cortical potentials during the attack. Cephalalgia. 1995;15:123–128. doi: 10.1046/j.1468-2982.1995.015002123.x. [DOI] [PubMed] [Google Scholar]
- Krymchantowski AV, Bigal ME, Moreira PF. New and emerging prophylactic agents for migraine. CNS Drugs. 2002;16:611–634. doi: 10.2165/00023210-200216090-00003. [DOI] [PubMed] [Google Scholar]
- Leao AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Ottman R, Ehrenberg DL, Hauser WA. Comorbidity of migraine: the connection between migraine and epilepsy. Neurology. 1994;44:S28–S32. [PubMed] [Google Scholar]
- Ludlow AK, Wilkins AJ, Heaton P. The effect of coloured overlays on reading ability in children with autism. J Autism Dev Disord. 2006;36:507–516. doi: 10.1007/s10803-006-0090-5. [DOI] [PubMed] [Google Scholar]
- Marcus DA, Soso MJ. Migraine and stripe-induced visual discomfort. Arch Neurol. 1989;46:1129–1132. doi: 10.1001/archneur.1989.00520460125024. [DOI] [PubMed] [Google Scholar]
- Newman Wright A, Wilkins A, Zoukos Y. J Neurol. 2007. Spectral filters can improve reading and visual search in patients with multiple sclerosis. (in press) [DOI] [PubMed] [Google Scholar]
- Nulty D, Wilkins AJ, Williams JM. Mood, pattern sensitivity and headache: a longitudinal study. Psychol Med. 1987;17:705–713. doi: 10.1017/s0033291700025940. [DOI] [PubMed] [Google Scholar]
- Padula WV, Shapiro JB. Head injury and the post-trauma vision syndrome. Review. 1993;244:153–158. [Google Scholar]
- Plant GT, Zimmern RL, Durden K. Transient visually evoked potentials to the pattern reversal and onset of sinusoidal gratings. Electroencephalogr Clin Neurophysiol. 1983;52:147–158. doi: 10.1016/0013-4694(83)90069-x. [DOI] [PubMed] [Google Scholar]
- Schoenen J. Deficient habituation of evoked cortical potentials in migraine: a link between brain biology, behavior and trigeminovascular activations? Biomed Pharmacother. 1996;50:71–78. doi: 10.1016/0753-3322(96)84716-0. [DOI] [PubMed] [Google Scholar]
- Singleton C, Henderson L-M. Computerised screening for visual stress in reading. J Res Read. 2007;30:316–331. [Google Scholar]
- Ts’o DY, Roe AW, Gilbert CD. A hierarchy of the functional organization for color, form and disparity in primate visual area V2. Vision Res. 2001;41:1333–1349. doi: 10.1016/s0042-6989(01)00076-1. [DOI] [PubMed] [Google Scholar]
- Tyrrell R, Holland K, Dennis D, Wilkins AJ. Colored overlays, visual discomfort, visual search and classroom reading. J Res Read. 1995;18:10–23. [Google Scholar]
- Van Harreveld A, Stamm JS. Cortical responses to metrazol and sensory stimulation in the rabbit. Electroencephalogr Clin Neurophysiol. 1955;7:363–370. doi: 10.1016/0013-4694(55)90005-5. [DOI] [PubMed] [Google Scholar]
- Welch KM. Contemporary concepts of migraine pathogenesis. Neurology. 2003;61:S2–S8. doi: 10.1212/wnl.61.8_suppl_4.s2. [DOI] [PubMed] [Google Scholar]
- Welch KM, D’Andrea G, Tepley N, Barkley G, Ramadan NM. The concept of migraine as a state of central neuronal hyperexcitability. Neurol Clin. 1990;8:817–828. [PubMed] [Google Scholar]
- Wilkins AJ. Visual stress. Oxford: Oxford University Press; 1995. [Google Scholar]
- Wilkins AJ. Coloured overlays and their effects on reading speed: a review. Ophthal Physiol Opt. 2002:448–454. doi: 10.1046/j.1475-1313.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ. Reading through colour. Chichester: John Wiley and Sons; 2003. [Google Scholar]
- Wilkins AJ, Huang J, Cao Y. Visual stress theory and its applicaton to reading and reading tests. J Res Read. 2004;27:152–162. [Google Scholar]
- Wilkins AJ, Lewis E. Coloured overlays, text and texture. Perception. 1999;28:641–650. doi: 10.1068/p2761. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Sihra N. A colorizer for use in determining an optimal ophthalmic tint. Color Res Appl. 2000;263:246–253. [Google Scholar]
- Wilkins AJ, Darby CE, Binnie CD. Neurophysiological aspects of pattern-sensitive epilepsy. Brain. 1979;102:1–25. doi: 10.1093/brain/102.1.1. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Binnie CD, Darby CE. Visually-induced seizures. Prog Neurobiol. 1980;15:86–117. doi: 10.1016/0301-0082(80)90004-0. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Nimmo-Smith MI, Tait A, McManus C, Della Sala S, Tilley A, Arnold A, Barrie M, Scott S. A neurological basis for visual discomfort. Brain. 1984;107:989–1017. doi: 10.1093/brain/107.4.989. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Nimmo-Smith MI. The clarity and comfort of printed text. Ergonomics. 1987;3012:1705–1720. doi: 10.1080/00140138708966059. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Nimmo-Smith I, Slater A, Bedocs L. Fluorescent lighting, headaches and eye-strain. Light Res Technol. 1989;21:11–18. [Google Scholar]
- Wilkins AJ, Evans BJW, Brown J, Busby A, Wingfield A, Jeanes R, Bald J. Double-blind placebo-controlled trial of precision spectral filters in children with reading difficulty. Ophthal Physiol Optics. 1994;14:365–370. [PubMed] [Google Scholar]
- Wilkins AJ, Jeanes RJ, Pumfrey PD, Laskier M. Rate of Reading Test: its reliability, and its validity in the assessment of the effects of coloured overlays. Ophthalmic Physiol Opt. 1996;16:491–497. [PubMed] [Google Scholar]
- Wilkins AJ, Baker A, Amin D, Smith S, Bradford J, Boniface S, Zaiwalla Z, Besag FMC, Binnie CD, Fish D. Treatment of photosensitive epilepsy using coloured filters. Seizure. 1999;8:444–449. doi: 10.1053/seiz.1999.0337. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Lewis E, Smith F, Rowland E. Coloured overlays and their benefits for reading. J Res Read. 2001;181:10–23. [Google Scholar]
- Wilkins AJ, Patel R, Adjamian R, Evans BJW. Tinted spectacles and visually sensitive migraine. Cephalalgia. 2002;22:711–719. doi: 10.1046/j.1468-2982.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Sihra N, Myers A. Increasing reading speed using colours: issues concerning reliability and specificity, and their theoretical and practical implications. Perception. 2005a;34:109–120. doi: 10.1068/p5045. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Sihra N, Nimmo-Smith I. How precise do precision tints have to be and how many are necessary? Ophthal Physiol Opt. 2005b;25:269–276. doi: 10.1111/j.1475-1313.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Smith J, Willison CK, Beare T, Boyd A, Hardy G, Mell L, Peach C, Harper S. Perception. 2007. Stripes within words affect reading. (in press) [DOI] [PubMed] [Google Scholar]
- Xiao Y, Wang Y, Felleman DJ. A spatially organized representation of colour in macaque cortical area V2. Nature. 2003;421:535–539. doi: 10.1038/nature01372. [DOI] [PubMed] [Google Scholar]
- Zeki S. A century of cerebral achromatopsia. Brain. 1990;113:1721–1777. doi: 10.1093/brain/113.6.1721. [DOI] [PubMed] [Google Scholar]


