Abstract
Living in an enriched environment (EC) during development enhances memory function in adulthood; living in an impoverished environment (IC) impairs memory function. Compounds previously demonstrated to improve memory among IC rats include CDP-choline and uridine monophosphate (UMP). Brain phosphatidylcholine (PC) synthesis utilizes both the uridine formed from the metabolism of exogenous CDP-choline and UMP, and the choline formed from that of CDP-choline. It also uses the polyunsaturated fatty acid (PUFA) DHA, a precursor for the diacylglycerol incorporated into PC. DHA administration also improves cognition in young and aged rodents and humans; its effects on cognitively-impaired IC rats have not been characterized. We have thus examined the consequences of administering DHA (300 mg/kg ) by gavage, UMP (0.5% in the diet), or both compounds on hippocampal- and striatal-dependent forms of memory among rats exposed to EC or IC conditions for 1 month starting at weaning, and consuming a choline-containing diet. We observe that giving IC rats either dietary UMP or gavaged DHA improves performance on the hidden version of the Morris water maze (all P<0.05), a hippocampal-dependent task; co-administration of both phosphatide precursors further enhances the IC rats’ performance on this task (P<0.001). Neither UMP, DHA, nor both compounds affects the performance of EC rats on the hidden version of the Morris water maze (P>0.05), nor the performance by IC or EC rats on the visible version of the Morris water maze (all P>0.05), a striatal-dependent task. We confirm that co-administration of UMP and DHA to rats increases brain levels of the phosphatides PC, PE, SM, PS, PI, and total brain phospholipid levels (all p<0.05), and show that rearing animals in an enriched environment also elevates brain PC, PS, and PI levels (all p<0.01) and total brain phospholipids (p<0.01) compared with their levels in animals reared in an IC environment. These findings suggest that giving DHA plus UMP can ameliorate memory deficits associated with rearing under impoverished conditions, and that this effect may be mediated in part through enhanced synthesis of brain membrane phosphatides.
Keywords: Choline, UMP, DHA Learning, Memory
Introduction
Changes in an animal’s environment, and the ways in which that animal reacts to those changes, can have significant long-term effects on the brain and behavior [1]. Laboratory rodents are normally reared under standard conditions (SC), during which they are housed individually and exposed to few novel objects. If instead the animals are reared under environmentally-enriched (EC) or impoverished conditions (IC), memory functions are improved in the former state [2] and impaired in the latter [3]. Rearing under EC conditions typically involves being housed in larger cages, with more cage mates, and novel objects; rearing under IC conditions typically involves being housed in smaller cages, isolation from other rodents, and exposure only to essential objects [4].
Environmental enrichment is known to enhance memory performance in various learning tasks, including performance by mice on a water maze test of spatial memory [5,6]; by rats on a T-maze [7]; on tests of spatial learning by adult and aged rats [8]; and of learning by rats with traumatic brain injury (TBI) [9]. The hippocampus, which is essential in learning and memory [1], is the brain region most profoundly affected by rearing in an enriched or impoverished environment [10].
Environmental impoverishment has been shown to impair memory function in a number of learning tasks, and various compounds reportedly protect rodents from these impairments. IC rats demonstrated deficits when tested on a T-maze [3] or using a water maze [11], and various hippocampal-dependent memory deficits [4]. Compounds previously demonstrated to improve memory in IC rats include CDP-choline and uridine mono-phosphate (UMP). Long-term dietary supplementation with either CDP-choline, a source of choline and circulating cytidine (in rats) or uridine (in humans), prevented memory impairment in IC rats tested on a hippocampal- dependent spatial task [12,13].
PC synthesis requires the use of three circulating compounds; choline; a pyrimidine such as uridine; and a polyunsaturated fatty acid (PUFA) such as docosahexaenoic acid (DHA) [14]. Consumption of DHA during development [15] or by aged rats [16] can improve learning and memory, and chronic administration of either the uridine source UMP, or especially, of DHA, can increase brain phosphatide levels [14].
Administration of these compounds or choline increases phosphatide synthesis by enhancing the substrate-saturation of low-affinity brain enzymes that catalyze their use in the Kennedy cycle. The first of these, choline kinase (CK), phosphorylates choline, to form phosphocholine. Uridine is phosphorylated by uridine–cytidine kinase (CDK) to form uridine triphosphate (UTP) [17], which is transformed in brain to cytidine triphosphate (CTP) by the enzyme CTP synthetase [18]. DHA is acylated by fatty acyl-CoA synthetase [19]. Phosphocholine and CTP combine to form cytidine-5′-diphosphocholine (CDP-choline), which then combines with diacylglycerol (DAG) to form phosphatidylcholine (PC) [20]. DAG species containing polyunsaturated fatty acids (PUFA) such as DHA are preferentially incorporated into PC [19,21]. All of these enzymes have high Km’s for their substrates relative to normal plasma and brain levels [18]. Uridine conversion to UTP and CTP is enhanced in PC12 cells [24] and rodent brain [14] when the saturation of uridine-cytidine kinase (UCK) [25,26,27,28,29,30] has been increased by pyrimidine administration [17]. Also, the concentration of CTP in brain is insufficient to saturate CTP: phosphocholine cytidylyltransferase (CT) [3,32]. Giving animals DHA, choline and uridine increases enzyme saturation by all three of these precursors, and thus maximally potentiates synthesis of PC and other phosphatides [14].
The increases in brain phosphatides caused by administering DHA alone or, especially DHA plus UMP are associated with increases in pre- and post-synaptic proteins [14], as well as in hippocampal dendritic spines [41], and probably, synapses. These additional hippocampal synapses could underlie enhancements in hippocampal types of memory. We thus examined the effects of DHA and UMP on hippocampal- and striatal-dependent forms of memory among rats that had been exposed to EC or IC for 1 month starting at weaning, using the hidden and visible versions of the Morris water maze. We also subsequently analyzed brain samples for their phospholipid contents and correlated the extent of each treatment on phosphatide levels and on performance on the water maze task.
Materials and Methods
Rats and Diet
Male Sprague Dawley rats, 4 weeks of age, purchased from Charles River Laboratories, Wilmington, MA, were housed in a climate-controlled area and exposed to a 12:12 hour light cycle (lights on at 7:00 h). The rats were matched according to body wt and assigned to either the IC or EC group. Subgroups of IC rats and EC rats were given either control diet, 0.5%; this diet supplemented with 300 mg/kg DHA daily by gavage, or 0.5% UMP and 300 mg/kg DHA daily by gavage. All diets also contained choline chloride. Rats were weighed weekly to ensure that animals exposed to each of the treatments were eating equivalent amounts of food. Efforts were made to minimize animal suffering, according to NIH guidelines. Protocols were approved by the Massachusetts Institute of Technology Institutional Animal Care and Use Committee (IACUC).
Environmental Conditions
Rats were housed in the same rack in plastic cages with wire lids. Bedding and water were regularly changed, and rats had ad libitum access to food and water. EC rats were housed in groups of 2, in cages containing plastic toys (blocks, balls, PVC tubing, etc.). Toys were rotated between groups twice a week; new toys were introduced weekly. EC rats were exposed to a “playroom” measuring 5 ft × 5 ft every other day for 45 min., and handled daily. The "playroom" contained several toys including plastic tubing, small balls, plastic boxes, wire brushes, and paper towels to shred. The IC rats were housed individually, without toys; handled 3 times/week; and allowed to exercise only 3 times/week for 15 min in an empty room measuring 5 feet × 5 feet, with only the experimenter present.
Water Maze Apparatus
The water maze was a galvanized circular tank, 6 feet in diameter and 2 feet in height. The tank was filled with water maintained at room temperature to a depth of 1 foot, and located in a dimly lit room containing several extramaze cues. Four starting positions were spaced around the perimeter of the tank to divide the pool into 4 equal quadrants. For the visible platform version of the water maze, a white flag attached to the top of the submerged platform protruded above the water surface. A video camera mounted directly above the maze was linked to a computer with video tracking software to automatically record the escape latency (time to reach the platform), distance traveled (length of swim path taken to find the platform), and swim speed of all rats (HVS Image).
Behavioral Test
All behavioral training was carried out as described previously [12,13] between 1400 and 1800 h, and each experiment was repeated at least 3 times and by at least 2 different experimenters who were blind to the treatments. Briefly, rats were given 4 trials/day for 4 days to locate the hidden platform (1 cm below the water surface), which, for each rat, remained in the same position (within 1 of 4 quadrants). If a rat did not find the platform within 90 sec, it was guided to the escape platform by the experimenter. After mounting the platform, rats were allowed to remain on it for 20 sec. Following each trial, rats were removed from the maze and placed in a holding cage for a 30 sec intertrial interval. On the fifth day of testing rats were given a probe test; the platform was removed, and the swim path and time spent in the quadrant of the pool that had previously contained the platform were measured over 60 sec.
In the visible version of the Morris water maze, rats were given 4 trials/day for 4 days to locate the visible platform, which was placed in a different quadrant on each of the 4 trials. If a rat did not escape within 90 sec, it was manually guided to the escape platform by the experimenter. After mounting the platform, rats remained on it 20 sec. Following each trial, rats were removed from the maze and placed in a holding cage for a 30 sec intertrial interval.
Rotarod apparatus and testing
The rotarod apparatus consisted of a 3.2-cm-diameter rod (RRAC-3002; O’Hara & Company, Tokyo, Japan). The rotarod test was performed according to the procedure described previously [34]. Rats were tested on the rotarod following the completion of all water maze testing. During the training period, rats were placed on the rod rotating at 4 rpm, and this speed was gradually accelerated to 40 rpm at an acceleration of 0.15 rpm/s. The latency to fall (retention time) was measured with a cutoff time of 4 min. Rats were trained for 3 consecutive days, receiving four trials per day with 1 h intertrial interval.
Sample Collection
Rat brains were obtained immediately following the conclusion of each behavioral test; animals were anesthetized using CO2; decapitated; and their brains dissected, weighed, and homogenized in distilled water such that each sample contained the same ratio of tissue to water; i.e., 20mg tissue:1mL water. Samples were stored at −80 C for further analysis.
Total DNA Assay
To determine the total DNA in each sample of homogenized brain tissue, previously described techniques [35] were used. DNA readings were compared with those of standards. Briefly, known standards were diluted 50 µg/mL in DNA buffer (50mM KPO4, 2mM EDTA, 250mM NaCl, pH=7.4); 10 µL of standards and of homogenized tissue were then placed in well plates (Falcon MicroTest 96-well Assay Plate, optilux Black). Hoechst solution was diluted to 1µg/µL in DNA buffer, and 200µL was added to standard- and sample-containing wells. Following a thirty-minute incubation at room temperature, the plate was read and analyzed on a Bio Rad microplate reader, model 550, using Ascent Software.
Total Protein Assay
To determine the total protein content of each sample, previously described techniques [35] were used. Readings of homogenized brain tissues were compared those of known BSA standards. Briefly, BSA standards and samples were added to well plates (clear Falcon Pro-Bind 96 well assay plate); CuSO4 solution was diluted 1:49 in bichinconinic acid and added to all standard- and sample-containing wells. Following a thirty minute incubation at room temperature and in the dark, the plate was read and analyzed on Thermo Labsystem Fluoroskan Ascent using Microplate Manager software.
Total Phospholipid Assay
Total phospholipid contents were determined by comparing the phosphorus levels in samples with those in potassium phosphate standards. Phosphatides were extracted using previously described methods (Folch et al., 1957): 1 mL homogenates were mixed with 3 mL of chloroform and methanol mixture (2:1 v/v) and vortexed for 30 seconds. After cooling on ice for 1 hour, the mixture was added to 1 mL deionized water, and then to 3 mL of chloroform and methanol (2:1 v/v). After remaining at −4C for 18–20 hours, the mixture was separated by centrifugation at 3500 rpm for fifteen minutes at 4C; 100 µL aliquots of the bottom phase was dried in a savant lyopholizer and then digested in 70% perchloric acid for 1.5 hours at 150 C. Phosphatides were measured as described previously (Ulus et al., 1989): 300 µL of 15% ascorbic acid and 200 µL of 5% ammonium molybdate were added to samples and standards. These remained at room temperature for thirty minutes, and were then read on a Perkin-Elmer Lambda 3B UV/VIS spectrophotometer. The absorbency reading of each sample was compared to the absorbency readings of the standards to determine the phospholipid content in samples. This value was then expressed per DNA or protein content.
Phospholipid Separation
To measure the amounts of individual phospholipids, previously described methods (Svanborg and Svennerholm, 1961) were used. Digested samples were separated using thin layer chromatography (TLC); 30 µL of each sample was spotted onto Alltech silica gel G channeled plates, placed in running buffer (30 mL chloroform, 34 mL ethanol, 30 mL triethylamine, and 8 mL water) for 1.5 hours, and visualized by spraying plates with petroleum ether containing 1,6-diphenyl-1,3,5-hexatriene and viewing under UV light. Bands corresponding to individual phospholipids were scraped, reconstituted in methanol, and dried overnight in a savant lyopholizer. Following this initial separation step, procedures used to measure each sample’s phosphorous contents were the same as those described for the total phospholipid assay above.
Data analysis
For all tests comparing 2 groups, two-tailed t-tests were used. For comparisons involving more than one factor, or comparing more than 2 groups, factorial ANOVA was used.
Results
Body wt
Body weight did not differ among animals in the various control or experimental groups (data not shown), indicating that rats were probably eating equivalent amounts of their diets.
Effects of UMP, DHA, and Environmental Conditions on Rats Performance on a Hippocampal-dependent Water Maze Test
All groups were able to learn the hidden version of the Morris water maze to some degree, showing a decrease in the number of errors recorded over time (figure 1A, B) and indicated by a significant main effect of day (block of 4 training trials per day) (P<0.001). Values are mean ± S.E.M, n=12 for each group. Main effects of environment (P<0.001) and of an environment × diet interaction were also observed (P<0.05). IC rats treated with either UMP, DHA, or UMP and DHA exhibited decreased escape latencies compared with those of IC control rats; the largest decrease was observed in IC rats receiving both UMP and DHA; UMP [F(1,12) = 7.563, P<.042], DHA [F(1,12) = 13.253. P <.035, and UMP × DHA [F(1,12) = 27.635, P<.001] (figure 1A). EC rats treated with either UMP, DHA, or UMP and DHA did not acquire the task at a faster rate then did control rats (P>0.05) (figure 1B). These results indicate that long-term dietary treatment with UMP, DHA or, especially UMP plus DHA improves the spatial memory deficits associated with impoverished conditions but does not affect this memory function in rats reared in an enriched environment.
Figure 1. Morris water maze - Hidden.
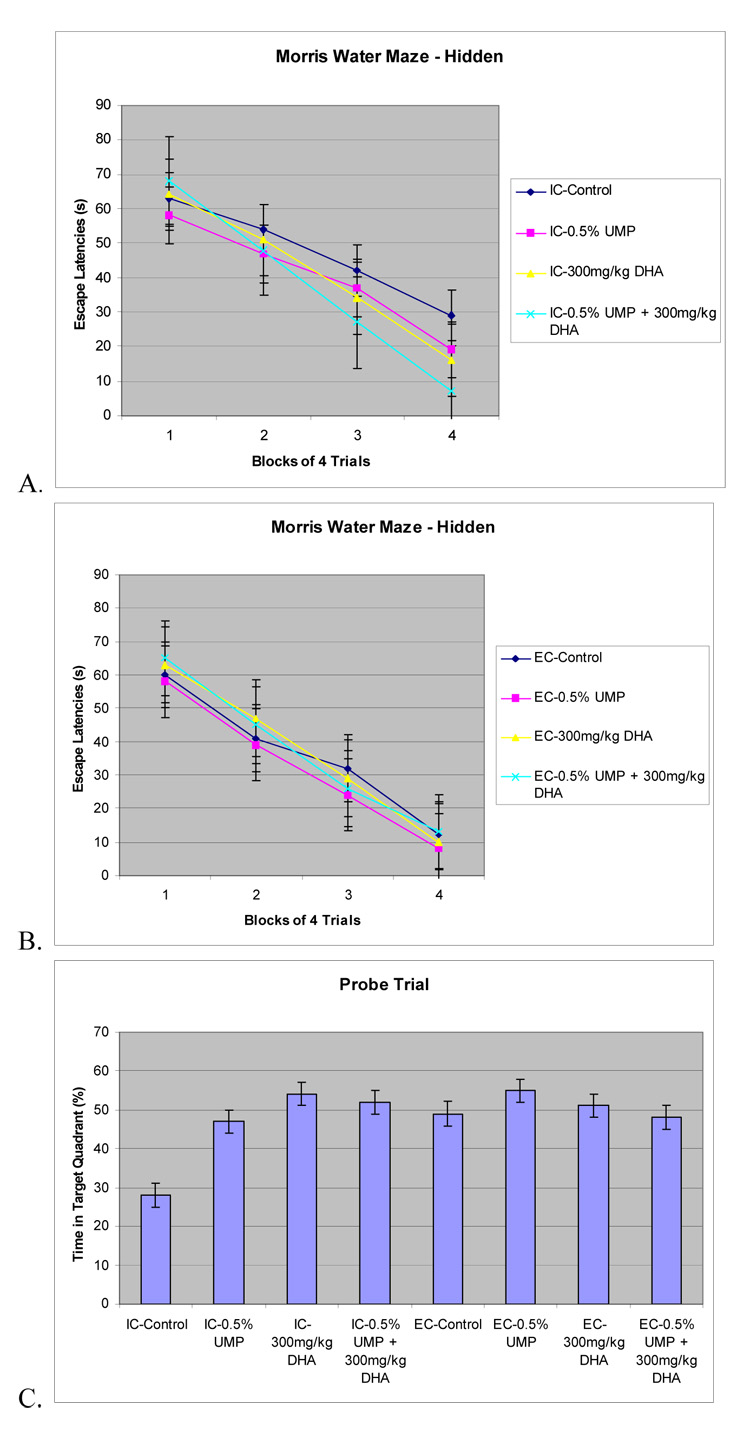
The effects of environment, UMP, and DHA administration on memory for a hippocampal-dependent hidden platform water maze in rats reared under EC or IC conditions for 1 month immediately postweaning. Values are means ± SEM, n=12. A. IC rats administered UMP, DHA, or UMP and DHA had decreased escape latencies compared to the IC control rats (all P< 0.05). B. EC rats administered UMP, DHA, or UMP and DHA did not have decreased escape latencies compared to the EC control rats (all P>0.05) C. The 60 sec probe test was effected by environment (P<0.042), quadrant (P<0.001), and diet × environment interaction (P<0.05).
The results of the 60 sec probe test indicated that all experimental groups spent more time in the quadrant that had originally contained the platform, suggesting that the rats used spatial cues to locate the hidden platform (figure 1C).The percentage of swim time each group spent was affected by whether it had been reared in an impoverished or exposed environment (P<0.042); quadrant (P<0.001); and diet × environment interaction (P<0.05). IC rats treated with UMP, DHA, or UMP plus DHA spent more time in the correct quadrant than did IC control rats not receiving either compound, UMP [F(1,12) = 7.845, P<0.025], DHA [F(1,12) = 12.374, P< 0.021], UMP × DHA [F(1,12) = 22.428, P<0.001) (figure 1C). In contrast, EC rats treated with UMP, DHA, or UMP and DHA did not spend more time in the correct quadrant than EC control rats (P>0.05).
Effects of UMP, DHA, and Environmental Conditions on Rats Performance on a Striatal-dependent Water Maze Test
All groups were able to learn the visible version of the Morris water maze to some degree, showing a decrease in the number of errors recorded over time (figure 2A, B) and a main effect of day (block of 4 training trials per day) (P<0.001). Values are means ± S.E.M, n=12. No other significant effects were observed, suggesting that environment or treatment with UMP or DHA have no effect on striatal-dependent learning and memory.
Figure 2. Morris water maze - Visible.
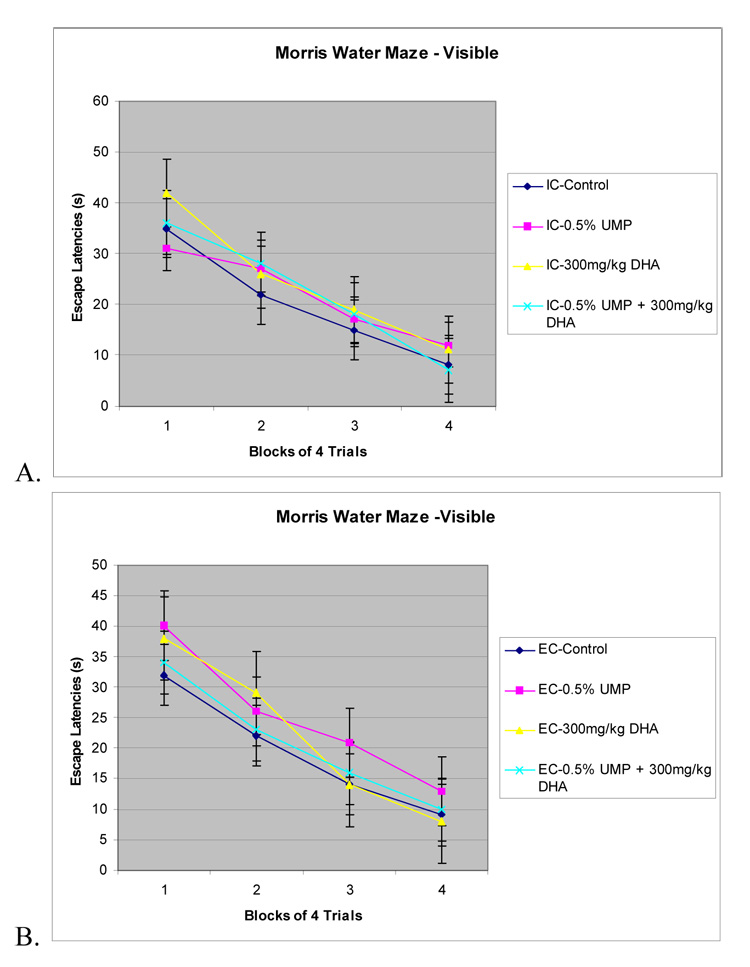
The effects of environment, UMP, and DHA administration on memory for a striatal-dependent visible platform water maze in rats reared under EC or IC conditions for 1 month immediately postweaning. Values are means ± SEM, n=12. A. IC rats administered UMP, DHA, or UMP and DHA did not have decreased escape latencies compared to the IC control rats (all P> 0.05). B. EC rats administered UMP, DHA, or UMP and DHA did not have decreased escape latencies compared to the EC control rats (all P>0.05).
Effects of UMP and DHA Supplementation on Rats Performance on an Accelerating Rotarod Test
All groups were able to learn the rotarod task, showing increases in the length of time they were able to remain on the accelerating rotarod (figure 3), as indicated by a significant main effect of day (block of four training trials/day) (p<.015). Values are mean ± S.E.M, n=12. No other significant effects were observed (p’s >.05), suggesting that environment or treatment with UMP or DHA have no effect on rat motor activity, per se.
Figure 3. Rotarod.
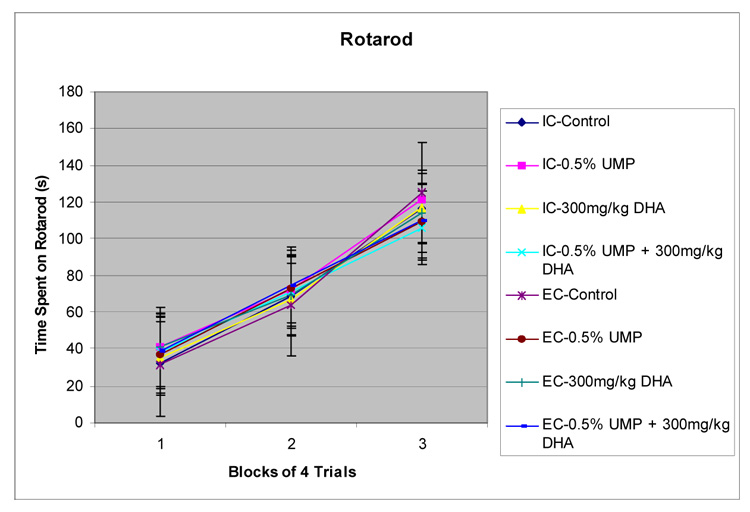
The effects of a UMP-supplemented diet and/or daily administration of DHA on IC and EC rats tested on an accelerating rotarod motor activity test. Values are mean ± S.E.M, n=12. The time spent on the rotarod was not affected (P’s.> .05)
Effects of a UMP- supplemented Diet alone or in Combination with DHA Administration on Brain Phosphatide Levels in EC and IC Rats
Chronic consumption of UMP (0.5%) increased IC rats’ brain PC, PE, PS, and PI levels significantly, by 23%, 28%, 46%, and 27%, respectively (table 1). Administration of DHA (300 mg/kg) to IC rats consuming control diet also increased rats’ brain PC, SM, PS, and PI levels significantly, by 26%, 49%, 71%, and 59%, respectively. Among IC rats receiving both UMP and DHA, brain PC, PE, SM, PS, and PI levels rose substantially more, i.e. by 60%, 97%, 86%, 138%, and 100%, respectively. Total phospholipids levels were also significantly increased, by 19% in IC rats receiving DHA and by 29%, in IC rats receiving both UMP and DHA (table 1). Two-way ANOVA revealed a significant effect of dietary UMP or oral DHA on IC rats brain PC, PE, PS, and PI levels (all P < .05). Two-way ANOVA also revealed a significant effect of DHA (p<.05) or of co-administering dietary UMP and oral DHA on phospholipids levels in brains of IC rats (p < .001). Similar results were obtained when data were expressed per µg DNA (data not shown).
Table 1.
Effects of giving UMP-supplemented diet (0.5%) and DHA (300 mg/kg) on phosphatide levels in whole brain samples.
| Treatment | Total PL (nmol/mg protein) | PC | PE | SM | PS | PI |
|---|---|---|---|---|---|---|
| IC-Control | 420 ± 9 | 168 ± 6 | 74 ± 7 | 49 ± 8 | 24 ± 4 | 22 ± 3 |
| IC-0.5% UMP | 442 ± 12 | 206 ± 8 | 95 ± 9* | 52 ± 6 | 35 ± 5*** | 28 ± 5* |
| IC-300mg/kg DHA | 501 ± 7* | 211 ± 11* | 78 ± 6 | 73 ± 5*** | 41 ± 5*** | 35 ± 5*** |
| IC-0.5% UMP + 300mg/kg DHA | 543 ± 15* | 269 ± 9*** | 146 ± 10*** | 91 ± 6*** | 57 ± 6*** | 44 ± 4*** |
| EC-Control | 524 ± 11a | 223 ± 8aaa | 88 ± 9 | 47 ± 5 | 30 ± 5a | 31 ± 6aa |
| EC-0.5% UMP | 529 ± 9 | 212 ± 12 | 98 ± 11 | 49 ± 7 | 31 ± 4 | 28 ± 4 |
| EC-300mg/kg DHA | 539 ± 14 | 227 ± 7 | 77 ± 5 | 78 ± 8*** | 39 ± 6** | 37 ± 7* |
| EC-0.5% UMP + 300mg/kg DHA | 542 ± 12* | 263 ± 11*** | 129 ± 8 | 94 ± 5 | 62 ± 4* | 48 ± 9*** |
IC and EC rats were administered a UMP-containing (0.5%) diet, and received DHA (300 mg/kg) daily by gavage for 6 weeks. Values are mean ± S.E.M, n=12. Brains were then obtained and their phosphatides levels determined as described in the text. Data are presented as nmol/mg protein.
P<.05 compared to control group
P<.01 compared to control group
P<.001 compared to control group
P<0.05 EC control compared to IC control
P<0.01 EC control compared to IC control
P<0.001 EC control compared to IC control
Administration of DHA (300 mg/kg) to EC rats consuming the control diet increased their brain levels of SM, PS, and PI significantly, by 66%, 30%, and 19%, respectively (table 1). Among EC rats receiving both UMP and DHA, brain PC, PE, SM, PS, and PI levels all rose significantly by 18%, 46%, 100%, 107%, and 55%, respectively. Total phospholipids levels were significantly elevated in EC rats co-administered UMP and DHA, but not in EC rats receiving UMP, DHA or the combination of UMP and DHA (all p<0.05 (table 1). Two-way ANOVA revealed a significant effect of oral DHA on EC rat brain SM, PS, and PI levels (all P < .05), and a significant effect of dietary UMP and oral DHA on EC rat brain PC, PE, PS, and PI levels (all P < .05). Similar results were obtained when data were expressed per µg DNA (data not shown).
Rearing in an enriched environment increased rat brain PC, PS, and PI levels by 57%, 25%, and 41%, respectively. Total phospholipid levels were also significantly increased in EC control rats, compared with those in IC control rats, by 25%. Two-way ANOVA revealed a significant effect of rearing in the enriched environment on EC rats' brain PC, PS, and PI levels (all p < 0.01), and of the enriched environment on phosphatide levels in brains of EC control rats compared with those in IC control rats (P<.01).
Discussion
These data show that administering UMP (0.5%) or DHA (300 mg/kg) to rats reared in an impoverished environment improves their performance on the Morris water maze, a hippocampal-dependent task; moreover co-administration of UMP and DHA further enhances this improvement, concurrently elevating brain levels of PC, PE, SM, PS, PI, and total brain phospholipids. In contrast, animals reared in an enriched environment exhibit no improvement in performance in response to UMP and/or DHA, and proportionately smaller increases in the phospholipids, primarily because control brain phospholipid levels in EC rats were higher than those in IC animals.
Among the many models of cognitive impairment available, we chose to use the environmental enrichment or impoverishment model because rats reared under IC exhibit cognitive deficits [3,4] that can be corrected in part by long-term dietary supplementation with either CDP-choline or UMP [12,13]. The cellular mechanisms associated with impaired cognition in IC rats are reportedly similar to those associated with CNS damage or degeneration [39]. Thus, compounds that can improve the memory of IC rats may also benefit patients with some types of neuronal damage [40].
We propose that UMP and DHA protect against cognitive impairment in IC rats by increasing the formation of neuronal membrane, specifically synaptic membrane, [14], and dendritic spines [41], and thereby promote neurotransmission. Rats reared under IC display reduced brain phosphatide levels (table 1), and reduced glutamatergic hippocampal transmission [3]. UMP and DHA administration increases brain phosphatide levels [14], and the density of hippocampal dendritic spines, [41] which require additional synaptic membrane. DHA administration reversed the age-related decline in GluR2 and NR2B glutamate receptor subunits, thereby improving glutamatergic transmission in the hippocampus [42]. In support of this proposed interpretation, we confirmed prior [14] findings that administration of UMP and DHA increases brain phosphatide levels (table 1) and concurrently protect IC rats from memory impairment (figure 1).
UMP and DHA may protect the brains of IC reared animals by restoring neuronal function to levels normally observed in brains of control or EC rats. Rats exposed to IC conditions [43] or made DHA-deficient [44] have decreased brain weight and size, while DHA administration increases brain weight and size [44]. Brains of IC reared rats also exhibit decreased neurogenesis [45] and synaptogenesis [46], DHA has been shown to promote neurite outgrowth in hippocampal neurons [47] and uridine promotes neurite outgrowth from PC12 cells [24]. DHA supplementation increased brain-derived neurotrophic factor (BDNF) levels in rats [48] while consuming a diet deficient in DHA decreased these levels [49]; BDNF induces neurogenesis in the hippocampal dentate gyrus [50]..
There are many similarities between the neural effects of rearing under EC and of chronically administering DHA, or UMP plus DHA. EC rodents exhibit improved spatial memory [8], while administration of DHA improves cognitive impairment in mouse models of mild cognitive dysfunction or in AD [51]. C57BL/6 learning-impaired mice [52] show increased survival of hippocampal cells if exposed to an EC, while DHA prolongs cell survival in retinal photoreceptors [53]. Brains of EC rodents exhibit increased NGF (nerve growth factor) expression [54]; likewise DHA administration can increase the expression of NGF [55]. Brains of EC rodents also exhibit increased expression of mRNA for BDNF [8]; BDNF modulates synapsin 1 levels during learning [56], and giving UMP plus DHA increases synapsin 1 levels in gerbil brain [14]. Brains of EC rodents have increased release of brain acetylcholine [57], while DHA supplementation increases potassium-evoked acetylcholine release [58], and UMP supplementation increases the basal and evoked release of acetylcholine [59].
In summary, the present study demonstrates that administration of UMP, DHA, or both, plus choline for 4 weeks prevents memory impairment in IC reared rats and increases brain levels of individual phosphatides and total phospholipids. The largest increase in phospholipids and greatest protection from memory impairments occur when choline, DHA, and UMP are administered in combination. EC has been implicated as a possible treatment for preventing memory impairment in such diseases as traumatic brain injury (TBI) [40,60], prenatal hypoxia [61], epilepsy [62], stroke [63], Huntington’s Disease [64,65] and depression [66]. Co-administration of DHA and UMP may aid in their treatment as well.
Acknowledgements
We thank Lisa Teather for advice and assistance in preparing this paper. We also thank Rona Stephanopoulos and Paul Jaffe for their assistance with behavior and biochemistry assays. This study was supported by NIH grant MH-28783 and the CBSMCT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 58.Aïd S, Vancassel S, Linard A, Lavialle M, Guesnet P. Dietary docosahexaenoic acid [22: 6(n-3)] as a phospholipid or a triglyceride enhances the potassium chloride-evoked release of acetylcholine in rat hippocampus. J Nutr. 2005;135:1008–1013. doi: 10.1093/jn/135.5.1008. [DOI] [PubMed] [Google Scholar]
- 27.Anderson E. Nucleoside and nucleotide kinases. In: Boyer P, editor. The Enzymes. New York: Academic Press; 1973. pp. 49–96. [Google Scholar]
- 7.Bernstein L. A study of some enriching variables in a free-environment for rats. J Psychosom Res. 1973 Mar;17:85–88. doi: 10.1016/0022-3999(73)90008-1. [DOI] [PubMed] [Google Scholar]
- 47.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 64.Dahlqvist P, Zhao L, Johansson IM, Mattsson B, Johansson BB, Seckl JR, Olsson T. Environmental enrichment alters nerve growth factor-induced gene A and glucocorticoid receptor messenger RNA expression after middle cerebral artery occlusion in rats. Neuroscience. 1999;93:527–535. doi: 10.1016/s0306-4522(99)00183-9. [DOI] [PubMed] [Google Scholar]
- 23.Cohen BM, Renshaw PF, Stoll AL, Wurtman RJ, Yurgelun-Todd D, Babb SM. Decreased brain choline uptake in older adults. An in vivo proton magnetic resonance spectroscopy study. JAMA. 1995;274:902–907. [PubMed] [Google Scholar]
- 56.Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 42.Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol Aging. 2007;28:424–439. doi: 10.1016/j.neurobiolaging.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Falkenberg T, Mohammed AK, Henriksson B, Persson H, Winblad B, Lindefors N. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is associated with improved spatial memory and enriched environment. Neurosci Lett. 1992;138:153–156. doi: 10.1016/0304-3940(92)90494-r. [DOI] [PubMed] [Google Scholar]
- 36.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 61.Foley AG, Murphy KJ, Regan CM. Complex-environment rearing prevents prenatal hypoxia-induced deficits in hippocampal cellular mechanisms necessary for memory consolidation in the adult Wistar rat. J Neurosci Res. 2005;82:245–254. doi: 10.1002/jnr.20641. [DOI] [PubMed] [Google Scholar]
- 52.Fordyce DE, Wehner JM. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain Res. 1993;619:111–119. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- 15.Gamoh S, Hashimoto M, Sugioka K, et al. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience. 1999;93:237–241. doi: 10.1016/s0306-4522(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 16.Gamoh S, Hashimoto M, Hossain S, Masumura S. Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clin Exp Pharmacol Physiol. 2001;28:266–270. doi: 10.1046/j.1440-1681.2001.03437.x. [DOI] [PubMed] [Google Scholar]
- 53.German OL, Insua MF, Gentili C, Rotstein NP, Politi LE. Docosahexaenoic acid prevents apoptosis of retina photoreceptors by activating the ERK/MAPK pathway. J Neurochem. 2006;98:1507–1520. doi: 10.1111/j.1471-4159.2006.04061.x. [DOI] [PubMed] [Google Scholar]
- 9.Giza CC, Griesbach GS, Hovda DA. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav Brain Res. 2005;157:11–22. doi: 10.1016/j.bbr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg N, Schumm DE, Webb TE. Uridine kinase activities and pyrimidine nucleoside phosphorylation in fluoropyrimidine-sensitive and –resistant cell lines of the Novikoff hepatoma. Biochem J. 1977;164:379–387. doi: 10.1042/bj1640379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greenough WT. In: Neural Mechanisms of Learning and Memory. Rosenzweig MR, Bennett EL, editors. Cambridge, Massachusetts: MIT Press; 1976. pp. 255–278. [Google Scholar]
- 66.Hattori S, Hashimoto R, Miyakawa T, Yamanaka H, Maeno H, Wada K, Kunugi H. Enriched environments influence depression-related behavior in adult mice and the survival of newborn cells in their hippocampi. Behav Brain Res. 2007;180:69–76. doi: 10.1016/j.bbr.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 1.Hebb DO. The Organization of Behavior. New York: Wiley; 1949. p. 335. [Google Scholar]
- 39.Horner PJ, Gage FH. Regenerating the damaged nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- 32.Houtsmuller UMT. Metabolic fate of dietary lecithin. In: Wurtman RJ, Wurtman JJ, editors. Nutrition and the Brain. Vol. 5. New York: Raven Press; 1979. pp. 83–94. [Google Scholar]
- 46.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J. Cereb. Blood Flow Meta. 1992;12:110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 63.Johansson BB. Functional outcome in rats transferred to an enriched environment 15 days after focal brain ischemia. Stroke. 1996;27:324–326. doi: 10.1161/01.str.27.2.324. [DOI] [PubMed] [Google Scholar]
- 5.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy EM, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipids. J Biol Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- 60.Kolb B, Gibb R. Environmental enrichment and cortical injury: behavioral and anatomical consequences of frontal cortex lesions. Cereb Cortex. 1991;1:189–198. doi: 10.1093/cercor/1.2.189. [DOI] [PubMed] [Google Scholar]
- 51.Kotani S, Sakaguchi E, Warashina S, Matsukawa N, Ishikura Y, Kiso Y, Sakakibara M, Yoshimoto T, Guo J, Yamashima T. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res. 2006 Oct;56(2):159–164. doi: 10.1016/j.neures.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 21.Marszalek JR, Kitidis C, DiRusso CC, Lodish HF. Long-chain acyl-CoA synthetase 6 preferentially promotes DHA metabolism. J Biol Chem. 2005;280:10817–10826. doi: 10.1074/jbc.M411750200. [DOI] [PubMed] [Google Scholar]
- 19.Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annu Rev Cell Dev Biol. 2005;21:633–657. doi: 10.1146/annurev.cellbio.21.122303.120624. [DOI] [PubMed] [Google Scholar]
- 33.McGahon B, Holscher C, McGlinchey L, Rowan MJ, Lynch MA. Training in the Morris water maze occludes the synergism between ACPD and arachidonic acid on glutamate release in synaptosomes prepared from rat hippocampus. Learn Mem. 1996;3:296–304. doi: 10.1101/lm.3.4.296. [DOI] [PubMed] [Google Scholar]
- 3.Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacolog. 2004;29:1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- 22.Millington R, Wurtman RJ. Choline administration elevates brain phosphorylcholine concentrations. J Neurochem. 1982;38:1748–1752. doi: 10.1111/j.1471-4159.1982.tb06658.x. [DOI] [PubMed] [Google Scholar]
- 44.Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc. Natl. Acad. Sci USA. 1986;83:4021–4025. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orengo A. Regulation of enzymic activity by metabolites. I. Uridine-cytidine kinase of Novikoff ascites rat tumor. J Biol Chem. 1969;244:2204–2209. [PubMed] [Google Scholar]
- 6.Pacteau C, Einon, Sinden J. Early rearing environment and dorsal hippocampal ibotenic acid lesions: long-term influences on spatial learning and alternation in the rat. Behav. Brain Res. 1989;34:79–96. doi: 10.1016/s0166-4328(89)80092-0. [DOI] [PubMed] [Google Scholar]
- 55.Pham TM, Söderström S, Winblad B, Mohammed AH. Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav Brain Res. 1999;103:63–70. doi: 10.1016/s0166-4328(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 24.Pooler AM, Guez DH, Benedictus R, Wurtman RJ. Uridine enhances neurite outgrowth in nerve growth factor-differentiated pheochromocytoma cells. Neuroscience. 2005;134:207–214. doi: 10.1016/j.neuroscience.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 57.Por SB, Bennett EL, Bondy SC. Environmental enrichment and neurotransmitter receptors. Behav. Neural Biol. 1982;34:132–140. doi: 10.1016/s0163-1047(82)91514-x. [DOI] [PubMed] [Google Scholar]
- 34.Powell CM, Schoch S, Monteggia L, Barrot M, Matos MF, Feldmann N, Sudhof TC, Nestler EJ. The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron. 2004;42:143–153. doi: 10.1016/s0896-6273(04)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 11.Renner MJ, Rosenzweig MR. Enriched and Impoverished Environments: Effects on Brain and Behavior. New York: Springer-Verlag; 1987. p. 134. [Google Scholar]
- 29.Ropp PA, Traut TW. Cloning and expression of a cDNA encoding uridine kinase from mouse brain. Arch Biochem Biophys. 1996;336:105–112. doi: 10.1006/abbi.1996.0537. [DOI] [PubMed] [Google Scholar]
- 30.Ropp PA, Traut TW. Uridine kinase: Altered enzyme with decreased affinities for uridine and CTP. Arch Biochem Biophys. 1998;359:63–68. doi: 10.1006/abbi.1998.0890. [DOI] [PubMed] [Google Scholar]
- 2.Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res. 1978;153:563–576. doi: 10.1016/0006-8993(78)90340-2. [DOI] [PubMed] [Google Scholar]
- 31.Ross BM, Moszczynska A, Blusztajn JK, Sherwin A, Lozano A, Kish SJ. Phospholipid biosynthetic enzymes in human brain. Lipids. 1997;32:351–358. doi: 10.1007/s11745-997-0044-x. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto T, Cansev M, Wurtman RJ. Oral supplementation with docosahexaenoic acid and uridine-5'-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. 2007;1182:50–59. doi: 10.1016/j.brainres.2007.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro LA, Ng KL, Zhou QY, Ribak CE. Olfactory enrichment enhances the survival of newly born cortical neurons in adult mice. Neuroreport. 2007;18:981–985. doi: 10.1097/WNR.0b013e3281532bc1. [DOI] [PubMed] [Google Scholar]
- 25.Skold O. Uridine kinase from Erlich ascites tumor: Purification and properties. J Biol Chem. 1960;235:3273–3279. [Google Scholar]
- 18.Spanner S, Ansell GB. Choline kinase and ethanolamine kinase activity in the cytosol of nerve endings from rat forebrain. Biochem J. 1979;110:201–206. doi: 10.1042/bj1780753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki NN, Koizumi K, Fukushima M, Matsuda A, Inagaki F. Structural basis for the specificity, catalysis, and regulation of human uridine-cytidine kinase. Structure. 2004;12:751–764. doi: 10.1016/j.str.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 38.Svanborg A, Svennerholm L. Plasma total lipids, cholesterol, triglycerides, phospholipids and free fatty acids in a healthy Scandinavian population. Acta Med Scand. 1961;169:43–49. [Google Scholar]
- 4.Teather LA, Magnusson JE, Chow CM, Wurtman RJ. Environmental conditions influence hippocampus-dependent behaviours and brain levels of amyloid precursor protein in rats. Eur J Neurosci. 2002;16:2405–2415. doi: 10.1046/j.1460-9568.2002.02416.x. [DOI] [PubMed] [Google Scholar]
- 12.Teather LA, Wurtman RJ. Dietary CDP-choline supplementation prevents memory impairment caused by impoverished environmental conditions in rats. Learn Mem. 2005;12:39–43. doi: 10.1101/lm.83905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teather LA, Wurtman RJ. Chronic administration of UMP ameliorates the impairment of hippocampal-dependent memory in impoverished rats. J Nutr. 2006;136:2834–2837. doi: 10.1093/jn/136.11.2834. [DOI] [PubMed] [Google Scholar]
- 37.Ulus IH, Wurtman RJ, Mauron C, Blusztajn JK. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res. 1989;484:217–227. doi: 10.1016/0006-8993(89)90364-8. [DOI] [PubMed] [Google Scholar]
- 10.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 43.Venable N, Pinto-Hamuy T, Arraztoa JA, Contador MT, Chellew A, Perán C, Valenzuela X. Greater efficacy of preweaning than postweaning environmental enrichment on maze learning in adult rats. Behav Brain Res. 1988;31:89–92. doi: 10.1016/0166-4328(88)90162-3. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Pooler AM, Albrecht MA, Wurtman RJ. Dietary uridine-5'-monophosphate supplementation increases potassium-evoked dopamine release and promotes neurite outgrowth in aged rats. J Mol Neurosci. 2005;27:137–145. doi: 10.1385/JMN:27:1:137. [DOI] [PubMed] [Google Scholar]
- 40.Will BE, Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Relatively brief environmental enrichment aids recovery of learning capacity and alters brain measures after postweaning brain lesions in rats. J Comp Physiol Psych. 1979;1:33–50. doi: 10.1037/h0077306. (1977) [DOI] [PubMed] [Google Scholar]
- 48.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21:1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 14.Wurtman RJ, Ulus IH, Cansev M, Watkins CJ, Wang L, Marzloff G. Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res. 2006;1088:83–92. doi: 10.1016/j.brainres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 62.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 65.Zhao LR, Mattsson B, Johansson BB. Environmental influence on brain-derived neurotrophic factor messenger RNA expression after middle cerebral artery occlusion in spontaneously hypertensive rats. Neuroscience. 2000;97:177–184. doi: 10.1016/s0306-4522(00)00023-3. [DOI] [PubMed] [Google Scholar]
- 54.Zhu SW, Yee BK, Nyffeler M, Winblad B, Feldon J, Mohammed AH. Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behav Brain Res. 2006;169:10–20. doi: 10.1016/j.bbr.2005.11.024. [DOI] [PubMed] [Google Scholar]


