Hermansky-Pudlak syndrome (HPS) is a rare autosomal recessive disease, characterized by a triad of oculocutaneous albinism (OCA), bleeding diathesis due to deficiency of dense bodies in platelets, and lysosomal accumulation of ceroid lipofuscin.1 Seven genetic subtypes of HPS have been identified in humans2; HPS1 on chromosome 10q23 is the most common and represents a founder effect in northwest Puerto Rico.1 The clinical features of HPS and its ophthalmic involvement3,4 are well documented, but no ocular histopathology has been published (to our knowledge). Herein, we describe the ocular histopathology of an adult patient with HPS type 1 (HPS-1).
Report of a Case
This study was approved by the National Human Genome Research Institute and National Eye Institute institutional review boards for human subjects, and informed consent was obtained from the patient. A 43-year-old Puerto Rican man was seen in March 2004. The diagnosis of HPS-1 was confirmed by demonstrating homozygosity for the 16–base pair duplication in exon 15 of HPS1. Systemic manifestations included OCA, colitis, nasal and gum bleeding, basal cell carcinoma, and severe pulmonary fibrosis.
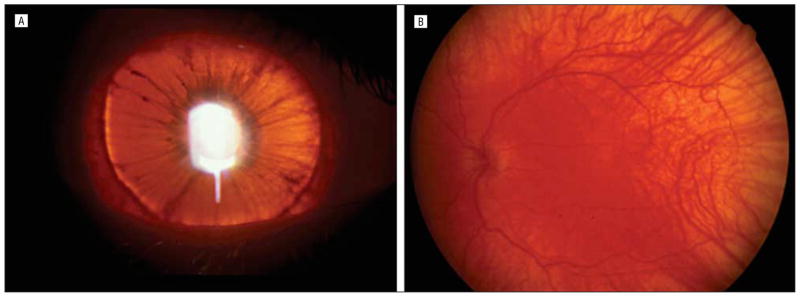
Findings from an ophthalmic examination revealed horizontal infantile-onset jerk nystagmus with a torsional component and intermittent exotropia. Best-corrected visual acuity was 20/160 OU (Early Treatment of Diabetic Retinopathy Study), and hyperopic astigmatism was present in both eyes. Posterior embryotoxon, marked iris transillumination, and macular transparency were noted (Figure 1). The absence of foveal pits and light reflexes indicated foveal hypoplasia, which was confirmed with optical coherence tomography (Figure 2). The patient died of pulmonary fibrosis in April 2005.
Figure 1.
Patient with Hermansky-Pudlak syndrome type 1 described herein. A, The right iris is light brown with marked transillumination such that the edge of the crystalline lens is clearly visible. B, The fundus of the left eye shows clear choroidal vasculature with residual pigmentation in the posterior pole.
Figure 2.
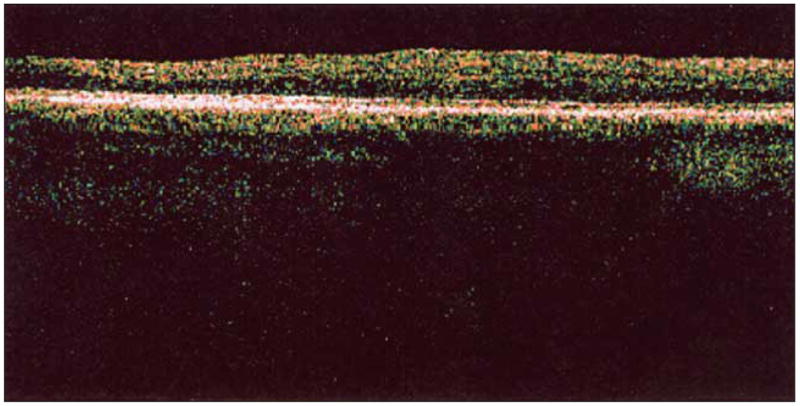
Optical coherence tomography with scan series through the macular area of the right eye retina demonstrates absence of foveal depression.
Pathologic Findings
Macroscopically, the right globe measured 24 × 25 × 22 mm and the left globe measured 23 × 24 × 23 mm. The cornea, anterior chambers, and optic nerves were normal. The uvea and retinal pigment epithelium displayed marked hypopigmentation. No macula lutea was visible. A focal hemorrhage was noted in the conjunctiva of the right eye.
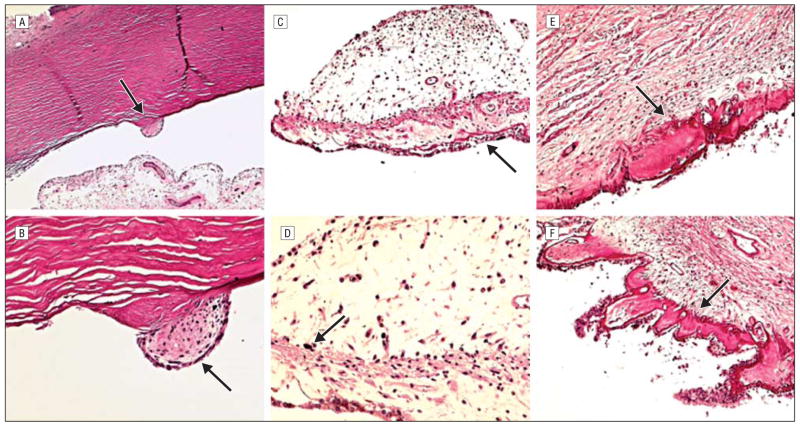
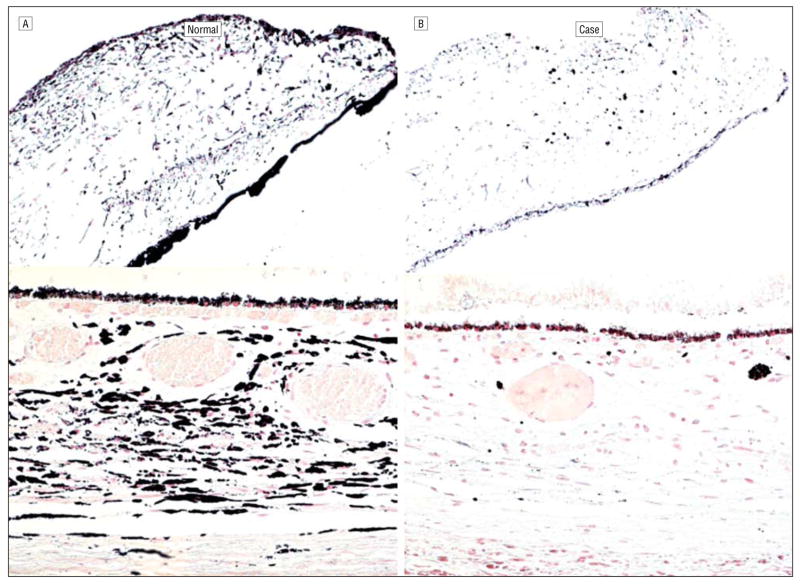
Microscopically, hemorrhage was present in the temporal conjunctiva of the right eye. A small cluster of mesenchymal cells was adherent to the enlarged and anteriorly located Schwalbe line (posterior embryotoxon) bilaterally (Figure 3A and B). The scleral spur of the right eye was hypoplastic. Only small aggregates of large melanin granules were visible in the pupillary margin (Figure 3C and D). Moderate hyalinization of the ciliary body was observed in both eyes (Figure 3E and F). There was marked depigmentation in the entire uvea (Figure 4). A few fine melanin granules remained in ocular pigment epithelial layers. The fovea showed a lack of differentiation, and the retinal pigment epithelium contained sparse melanin granules (Figure 4B). A few hemorrhages and platelet aggregates were seen in the optic nerve head of the right eye.
Figure 3.
Patient with Hermansky-Pudlak syndrome type 1 described herein. A, Schwalbe ring (arrow) is prominently enlarged and displaced anteriorly (posterior embryotoxon). B, A small cluster of mesenchymal cells (arrow) is adherent to the prominent Schwalbe ring. C, Iris melanocytes are generally absent. Iris pigmented epithelial cells show loss of melanin granules resulting in a lacy appearance (arrow). D, Only small aggregates of large melanin pigment granules (arrow) were disclosed in the iris stroma at the pupillary margin. E and F, Moderate hyalinization (arrows) of the ciliary body was seen in both eyes (hematoxylineosin, original magnification ×50 [A], ×200 [B and D], and ×100 [C, E, and F]).
Figure 4.
Pigmentation comparisons. A, Healthy white subject with brown irises. B, Patient with Hermansky-Pudlak syndrome type 1 described herein showing paucity of pigmentation of the iris, choroid, and retinal pigment epithelium (Fontana-Masson, original magnification ×100 [iris] and ×200 [choroid]).
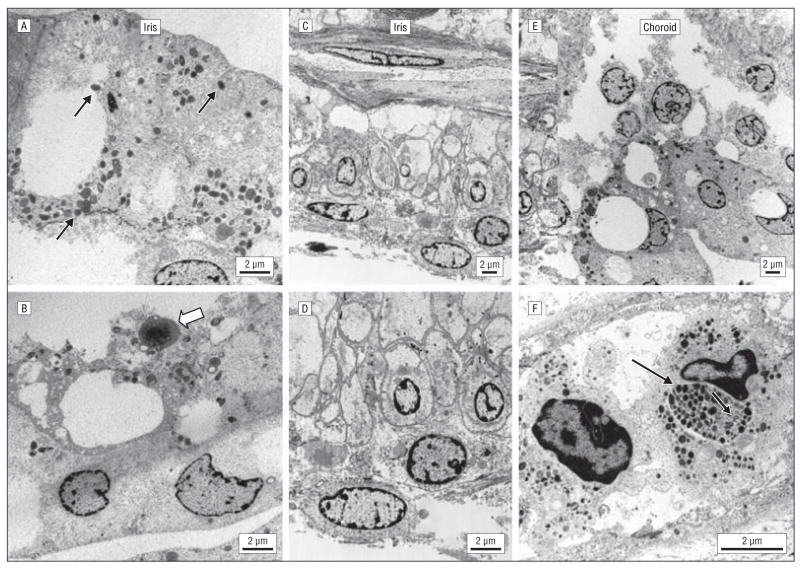
Ultrastructurally, the uveal tissues contained a few melanocytes with sparse immature melanosomes of incomplete round and oblong-shaped melanized granules (Figure 5A and B). Some melanocytes had electron-dense halos and electron-lucent cores. Most melanocytes were swollen and devoid of melanosomes. The nuclei had condensed chromatin, and some had a folded nuclear membrane. The iris pigmented epithelial cells were enlarged, with few cytoplasmic microorganelles (mitochondria, ribosomes, endoplasmic reticulum, andGolgi complex) and thickened basement membranes (Figure 5C and D). The morphologic structure of the choroid was similar to that of the iris (Figure 5E). Only rarely did choroidal melanocytes contain stage IV elanosomes (Figure 5F).
Figure 5.
Transmission electron micrographs. A, A few melanocytes that contained sparse immature stage III melanosomes (0.2–0.6 μm in diameter) with incomplete round and oblong melanized granules (arrows). B, Rare membrane-limited lipofuscinlike material with fat globules (open arrow) is noted. C and D, The iris pigmented epithelial cells are enlarged, contain a decreased number of cytoplasmic microorganelles, and have thickened basement membranes. E and F, In the choroids, many melanocytes were swollen and degenerated. Some melanocytes contained sparse, small, immature melanosomes, and some contained an aggregation of stage IV melanosomes (arrow). U-shaped structures were occasionally seen (open arrow) (scale bar, 2 μm).
Comment
Hermansky-Pudlak syndrome represents a disorder of the formation, sorting, or trafficking of intracellular vesicles, including melanosomes in melanocytes and dense bodies in platelets.5 As a consequence, patients with HPS-1 exhibit albinism, bleeding, and (for unknown reasons) pulmonary fibrosis and ceroid lipofuscinosis. The eyes of our patient had no ceroid accumulation, but their choroidal melanocytes contained numerous aberrant membranous structures, both U- and ring-shaped (Figure 5), as previously reported in HPS skin melanocytes.6,7 This is consistent with dysregulated targeting or fusion of Golgi-derived vesicles and with impaired targeting of melanocyte-specific proteins (eg, tyrosinase-related protein 1 and granulophysin) to premelanosomes.6
Other major ocular pathologic findings consisted of posterior embryotoxon with a small cluster of adherent mesenchymal cells, moderate hyalinization of the ciliary body, foveal hypoplasia, multiple ocular hemorrhages, and marked hypopigmentation. The iris pigment epithelium displayed the most severe ultrastructural pigmentary defect. Uveal melanocytes of the oculartissue contained mainly stage III melanosomes; the existence of stage IV melanosomes in rare choroidal melanocytes indicates that this patient with HPS-1 was still able to produce morphologically mature melanosomes, albeit in an extremely reduced quantity.
Hyalinization of the ciliary body occurs in aging ciliary processes, long-standing glaucoma, or heterochromic iridocyclitis, implying an atrophictendency. We propose that the hemorrhagic diathesis may have contributed to this complication. Axenfeld anomaly and foveal hypoplasia, also present in our patient, are common in OCA.8 Posterior embryotoxon was clinically apparent in 5 of 20 previously described patients with HPS, with only 1 manifesting iris process adhesion.3 In our patient, the mesenchymal cells, not the iris processes, were adherent to posterior embryotoxon. Such a subtle abnormality is difficult to detect clinically, especially in the presence of the underlying nystagmus of HPS.
Our patient’s ocular melanocytes contained mainly stage III and rarely stage IV melanosomes, consistent with previous reports of OCA.9 This finding differs, however, from descriptions of HPS skin melanocytes, which contain exclusively premature melanosomes, mostly stage I and II.6,9 Natsuga et al7 identified stage IV melanosomes in the melanocytic nevus of a patient with HPS-1 and hypothesized that this reflected the high melanin production of the nevus cells. The replacement of melanosomes with multiple vacuoles and a marked decrease in microorganelles of the iris pigmented epithelia have not been previously reported in OCA, to our knowledge. Although the occurrence of a giant melanosome in OCA and HPS is not unusual,7,9 no ultrastructural giant melanosomes were apparent in this case. Instead, large melanin granules were present in the pupillary margin microscopically, which could be pigmented macrophages.
The mouse pale ear (ep) mutation is the homologue of human HPS1.10 These mice exhibit abnormalities in melanosomes and platelet-dense granules that are similar to those in patients with HPS. Similar to our patient, ep mice initially have markedly decreased and unevenly pigmented melanosomes, lightly pigmented intermediate structures, and stage IV melanosomes. However, ep mice display macromelanosomes, which were absent in our patient.10
This case offers the first example (to our knowledge) of ocular histopathologic features in HPS-1. Similar descriptions are needed for the other HPS subtypes.
Acknowledgments
We acknowledge and thank Pamela C. Sieving, MA, MS, for her assistance and expertise in conducting an extensive and exhaustive literature search for this article.
Funding/Support: This study was supported by the National Institutes of Health/National Eye Institute Intramural Research Program.
Footnotes
Financial Disclosure: None reported.
References
- 1.Gahl WA, Brantly M, Kaiser-Kupfer MI, et al. Genetic defects and clinical characteristics of patients with a form of oculocutaneous albinism (Hermansky-Pudlak syndrome) N Engl J Med. 1998;338:1258–1264. doi: 10.1056/NEJM199804303381803. [DOI] [PubMed] [Google Scholar]
- 2.Gunay-Aygun M, Huizing M, Gahl WA. Molecular defects that affect platelet dense granules. Semin Thromb Hemost. 2004;30:537–547. doi: 10.1055/s-2004-835674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summers CG, Knobloch WH, Witkop CJ, Jr, King RA. Hermansky-Pudlak syndrome: ophthalmic findings. Ophthalmology. 1988;95:545–554. doi: 10.1016/s0161-6420(88)33152-0. [DOI] [PubMed] [Google Scholar]
- 4.Tsilou ET, Rubin BI, Reed GF, et al. Milder ocular findings in Hermansky-Pudlak syndrome type 3 compared with Hermansky-Pudlak syndrome type 1. Ophthalmology. 2004;111:1599–1603. doi: 10.1016/j.ophtha.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 5.Huizing M, Boissy RE, Gahl WA. Hermansky-Pudlak syndrome: vesicle formation from yeast to man. Pigment Cell Res. 2002;15:405–419. doi: 10.1034/j.1600-0749.2002.02074.x. [DOI] [PubMed] [Google Scholar]
- 6.Boissy RE, Zhao Y, Gahl WA. Altered protein localization in melanocytes from Hermansky-Pudlak syndrome: support for the role of the HPS gene product in intracellular trafficking. Lab Invest. 1998;78:1037–1048. [PubMed] [Google Scholar]
- 7.Natsuga K, Akiyama M, Shimizu T, et al. Ultrastructural features of trafficking defects are pronounced in melanocytic nevus in Hermansky-Pudlak syndrome type 1. J Invest Dermatol. 2005;125:154–158. doi: 10.1111/j.0022-202X.2005.23743.x. [DOI] [PubMed] [Google Scholar]
- 8.van Dorp DB, Delleman JW, Loewer-Sieger DH. Oculocutaneous albinism and anterior chambre cleavage malformations: not a coincidence. Clin Genet. 1984;26:440–444. doi: 10.1111/j.1399-0004.1984.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 9.Witkop CJ, Jr, Hill CW, Desnick S, et al. Ophthalmologic, biochemical, platelet, and ultrastructural defects in the various types of oculocutaneous albinism. J Invest Dermatol. 1973;60:443–456. doi: 10.1111/1523-1747.ep12702920. [DOI] [PubMed] [Google Scholar]
- 10.Gardner JM, Wildenberg SC, Keiper NM, et al. The mouse pale ear (ep) mutation is the homologue of human Hermansky-Pudlak syndrome. Proc Natl Acad Sci U S A. 1997;94:9238–9243. doi: 10.1073/pnas.94.17.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]