Abstract
It is widely thought that regulation of post-synaptic AMPA receptors is a critical component in changes in synaptic efficacy underlying learning and memory. The regulation of AMPA receptors occurs through trafficking of the receptor and/or modulation of the receptor’s channel properties and both of these processes depend on phosphorylation of the receptor. Using homologous recombination (knockin) techniques we targeted two phosphorylation sites on the AMPA-GluR1 receptor: the CaMKII/PKC Ser 831 site and the PKA Ser 845 site. Mice with both or either of these sites mutated were then tested on an incentive learning task that assessed their ability to acquire a simple association between a cue and reward and to then use this cue as a reinforcer to guide their behavior (conditioned reinforcement). We report that, whereas WT mice showed enhanced responding for the reward-associated cue, mice with mutations of both phosphorylation sites or the Ser 831 site alone, failed to show such a conditioned reinforcement effect. By contrast, mice with only the Ser 845 site deficient showed normal CS+-reinforced responding. Thus, action at the Ser 831 phosphorylation site was necessary for normal conditioned reinforcement. Finally, the behavioral deficit was highly specific: performance on a number of other measures of motivated performance, including responding reinforced by the food itself, was unaffected by the mutations. Our findings provide novel evidence for a molecular mechanism in a form of appetitive incentive learning critical in regulating normal motivated behavior, as well as maladaptive forms such as addiction and eating disorders.
Keywords: Conditioned reinforcement, glutamate, synaptic plasticity, transgenic mice
Glutamatergic AMPA receptors (AMPRs) mediate the majority of fast excitatory neurotransmission in the central nervous system. Recent studies have implicated their role in experience-dependent synaptic plasticity (neuroplasticity) underlying learning and memory [reviewed by, 1]. In hippocampus and cortex, for instance, long-term potentiation (LTP) and depression (LTD) - two well-established models of synaptic plasticity- are associated with changes in synaptic targeting of the receptor and ion-channel conductance. Additionally, mice with targeted mutations of the entire AMPA-GluR1 subunit (gria1) fail to show hippocampal (CA1) LTP [2] and show retention deficits on some hippocampal-dependent memory tasks [but see2, e.g. 3].
The regulation of AMPA receptors occurs through direct protein phosphorylation of the receptor, in particular, at two serine sites on the c-terminus domain of the GluR1 subunit; a Ser 831 which is phosphorylated by Ca(2+)/Calmodulin-dependent protein kinase II (CaMKII) or Protein Kinase C (PKC), and a Ser 845 site phosphorylated by Protein Kinase A (PKA) [4, 5]. Using mice with mutations that rendered both of these phosphorylation sites ineffective, Lee et al [6] have shown that phosphorylation of the AMPA receptor GluR1 subunit is critical for hippocampal CA1 LTP and LTD, as well as for long-term retention on a spatial memory task. These findings point to a critical role for direct phosphorylation of the GluR1 subunit in the synaptic plasticity underlying learning and memory.
Although the role of AMPA GluR1 regulation in hippocampal synaptic plasticity and hippocampal-dependent learning and memory is now relatively well characterized, much less is known about other forms of learning and memory that do not rely on hippocampal circuitries. Nonetheless, GluR1-containing AMPA receptors are found in many neurons throughout the brain. Thus, synaptic plasticity outside the hippocampus and forms of learning and memory that do not require hippocampal function may also depend on regulation of AMPA GluR1 receptor. Consistent with this idea, Rumpel et al [7] recently reported that, in the lateral amygdala, cued-fear conditioning was closely associated with synaptic incorporation of GluR1 receptors. Additionally, gria1 mutant mice show deficits on behavioral tasks of acquired affect and motivation that are known to depend on normal function in a circuit that includes the basolateral amygdala (BLA), ventral striatum, and prefrontal cortex [8, 9].
Here we assessed the role of AMPA GluR1 phosphorylation sites in the learning and memory process(es) through which otherwise neutral cues are able to serve as goals themselves (conditioned reinforcement). To this end, we used mutant mice in which the s831 and/or s845 GluR1 phosphorylation sites were targeted, either alone or in combination, using a gene knock-in technique to prevent phosphorylation at these sites. We report that it is the Ser 831, and not the ser 845 site on the AMPA GluR1 receptor, that is essential for Pavlovian cues to serve as conditioned reinforcers, an important hippocampus-independent learning function.
Methods and Procedures
Generation of mice
Double and single GluR1 Ser 831 and Ser 845 mutant mice were generated using PCR mutagenesis [6]. Briefly, a short genomic DNA fragment including exon 17 of mouse GluR1 was subcloned into pBR322 and alanine substitutions were introduced into 831 serine and 845 serine sites by PCR mutagenesis. Mutated fragments were introduced into the targeting vector in addition to neo resistance gene cassette (neor) with lox-P sequences at both sides. Correct homologous recombinant ES cells were screened by PCR and confirmed by Southern blot analysis. After germline transmission of the mutated GluR1 gene containing 831 alanine and 845 alanine mutations, neor was eliminated by breeding to Cre transgenic mice [10]. Cre transgene was bred out from the line and heterozygous mice without Cre gene were used for breeding to produce littermates for these experiments. Mutations of 831 and 845 phosphorylation sites were verified by Western blot analysis of hippocampal samples using phosphospecific antibodies (Figure 1). Verification of phosphorylation activity in double phosphorylation mutant mice was previously reported [6]. After confirmation, heterozygous mice were bred to produce homozygotes. For all experiments, mice with 129 and C57BL/6 hybrid genetic background were used.
Figure 1. Immunoblot blot analysis of phosphorylation activity in single site mutant mice.
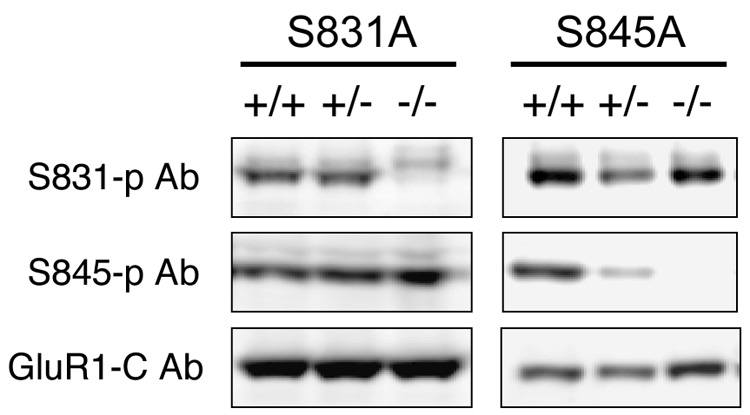
To verify the mutation, hippocampal samples from wild-type (+/+), heterozygote (+/−), and homozygote (−/−) were prepared for immunoblot (Western) analysis using phosphorylation sitespecific Ab's against S831 (top) and S845 (middle) or GluR1-carboxy tail Ab (bottom). Note that S831 and S845 phosphorylation signal is absent in S831 and S845 homozygotes, respectively, while phosphorylation at the alternate site and GluR1 expression levels (GluR1-C) are normal.
Housing and feeding
All mice were housed in groups of 4/cage in a light-cycle (12:12hr light:dark) and climate controlled facility in accordance with NIH and Johns Hopkins University standards. Male mice between 3 and 6 months of age were used in all experiments. Starting no less than 3 days prior to training for conditioned reinforcement, mice were food-restricted to about 85–90% of their free-feeding weight and this food restriction was maintained for the duration of training and testing for conditioned reinforcement. Behavioral testing was conducted during the light phase of the day.
Apparatus
All training and testing was conducted in standard mouse operant chambers (ENV-307W, Med Associates Inc. St. Albans, VT) located inside sound attenuating enclosures. A fan provided constant ventilation and low-level background noise (~75dB) to each chamber and an incandescent house light provided low-level (~200 Lux) white illumination. Each chamber was equipped with a motorized dipper mechanism that delivered a sweetened liquid reward solution (30% condensed milk in the Ser845 and double mutant conditions or 10% sucrose in the Ser 831 condition) in 0.01-ml volumes into a recessed receptacle. A speaker was located on the wall opposite the food cup and was used to present auditory stimuli.
Behavioral Procedures
Conditioning-reinforcement
Mice first received 2 × 40-min sessions designed to train them to approach the food cup and consume the sweetened liquid reward that served as the unconditioned stimulus (US). Next, mice were given Pavlovian discrimination training sessions intended to establish an association between an auditory stimulus (CS+) and the sweetened reward US. A second auditory stimulus was presented with equal frequency and probability but was not paired with liquid reward (CS−). The CSs were an 85dB, 2 kHz tone or an 80 dB white noise (counterbalanced) and were each 10 sec in duration. The sweetened liquid reward presentation coincided with the last 5-sec of the CS+ presentation. On each training session, 20 CS+ and CS− trials were presented in random order, on average every 60 sec - i.e., on a variable time schedule (range 30 – 90 sec). Once reliable and robust performance was established during the Pavlovian conditioning phase, a 40 min test for conditioned reinforcement was conducted. For the test, two nose-poke ports were mounted in the chamber on each side, and at equal distance of the food cup. Responding into one port resulted in a 3-sec presentation of the CS+ and responding into the alternate port resulted in a 3-sec presentation of the CS−. Importantly, mice were tested under extinction conditions and no rewards were present at any time during the test. Responses into the CS+ and CS− reinforced nose-poke ports, food-cup entries and overall locomotor activity levels were recorded onto a Windows-based PC running Med-PC IV.
Primary (food) reinforcement
In a previous experiment, these mice were trained to instrumentally respond for food reinforcement in identical mouse operant chambers, but this time outfitted with two retractable levers. The levers were located at equal distance and on either side of the recessed food magazine. For these experiments, the same WT and phosphorylation site mutant mice and the same liquid food reinforcers were used as during the Pavlovian phase of the conditioned reinforcement experiments. Importantly, mice were able to respond for the same primary reinforcer using the same continuous schedule of reinforcement (FR1) as was used during the test for conditioned reinforcement. Thus, during 2 consecutive 30-min sessions, each response on one lever (the active) lever delivered liquid reward delivery and responses on the second (control) lever had no programmed consequences. Active and inactive lever responses, liquid reward magazine entries and overall locomotor activity levels were recorded.
Data analysis
In all cases, the results were analyzed for each mutation condition (Double, 845 or 831) separately. Appetitive pavlovian training performance (food cup entries) was analyzed for significant effects of Session, CS (CS+ versus CS−) and/or Genotype (WT versus mutant) using 3-way analysis of variance (ANOVA). Instrumental performance on the test for CRf was compiled and analyzed using two-way ANOVA for significant effects of Cue (CS+ versus baseline/CS−) and Genotype. When appropriate, specific comparisons were conducted using Bonferroni corrected post-hoc analyses to determine within genotype differences in response rates for CS+ versus CS−. Additionally, the results from the CRf test were analyzed by calculating discrimination ratios (CS+ reinforced nose-pokes/total nose-pokes) and, for each group, comparing these values against a value of 0.5 (i.e., no discrimination) using a one-sample t-test. Additionally, discrimination ratios were compared for statistical differences as a function of genotype (WT versus mutant) for each phosphorylation condition. Locomotor activity and food cup responses during the test for conditioned reinforcement were analyzed for genotype difference using one-way ANOVAs. Active and control lever response rates during training sessions for liquid reward delivery were analyzed separately using two-way ANOVAs for significant effects of session and genotype.
Results
Conditioned reinforcement
Figure 2 (left side) shows the behavioral performance of mice with both AMPA GluR1 Ser 831 and 845 (Double) phosphorylation sites mutated (panel a), or mice with either phosphorylation sites mutated (panels c and e), on the Pavlovian discrimination training sessions. In all three genotype conditions, over the course of Pavlovian training (panels a, c and e), all groups showed more entries into the food cup during CS+ presentations than during CS− presentations. Thus, 3-way ANOVA resulted in significant interaction effects of Session × CS for all three genotype conditions (Fs > 3.3, ps < 0.001). Importantly, no WT versus mutant differences were evident, indicated by no main (Fs < 2.1, Ps > 0.18) or interaction effects with Genotype (Fs < 1.6, Ps > 0.1).
Figure 2. Pavlovian discrimination training and test for conditioned reinforcement in GluR1 phosphorylation site mutants.
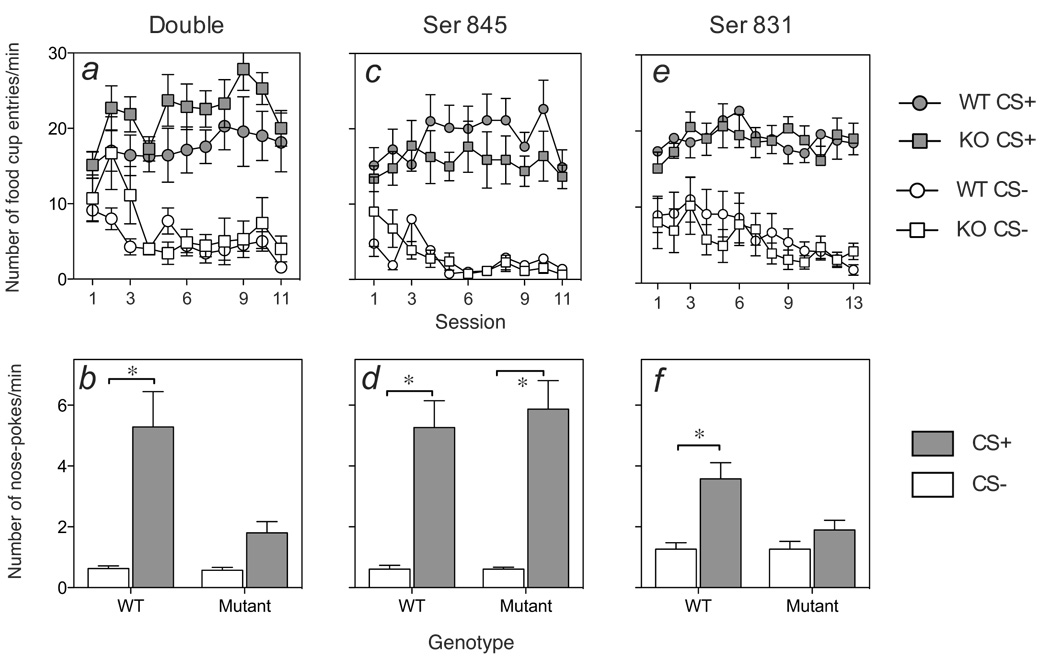
Results of the Pavlovian discrimination training sessions (numbers of food receptacle entries during the CS+ and CS− periods) and conditioned reinforcement test of incentive learning (CS+ and CS− reinforced nose poking) in mice with knock-in mutations of both Ser 845 and Ser 831 phosphorylation sites or littermate WT control mice (mutant N = 7, WT N = 6; panels a and b), or single-point mutations of the Ser 845 (mutant N = 8, WT N = 8; panels c and d) or Ser 831 (mutant N = 11, WT N = 12; panels e and f) site. The error bars show ± SEM.
Figure 2 (right side) shows performance on the subsequent session for conditioned reinforcement to test whether WT and phosphorylation mutant mice would perform an operant nose-poke response to receive presentations of the Pavlovian CS+ (panels b, d and f). WT mice showed greater preferential responding for the Pavlovian CS+ than either double (Ser 845 & 831) or Ser 831 phosphorylation site mutants (panels b, f). For both conditions, 2-way ANOVA showed significant main effects of Stimulus (Fs > 26.2, Ps < 0.001) and Genotype (Fs > 5.7, Ps < 0.05) as well as a significant interaction of Stimulus × Genotype (Fs > 8.8, Ps < 0.05). Indeed, based on subsequent post-hoc analyses the latter groups failed to show enhanced responding for the CS+ relative to baseline (CS−) response levels (panels b and f; Ps > 0.05). By contrast, both WT and mutant mice lacking only the Ser 845 phosphorylation site (panel d) showed robust and preferential responding on the CS+ reinforced nose-poke port, and these groups did not differ. Thus, a 2-way ANOVA yielded a significant effect of Stimulus (CS+ versus CS−; F(1,14)=57.8, p < 0.0001), but no effect of Genotype or Stimulus × Genotype interaction (Fs < 0.22, ps > 0.64).
Table 1 shows the calculated discrimination ratios (DRs) between CS+ versus CS-reinforced nose-poke responses during the test for conditioned reinforcement for each group. In all three conditions, WT mice showed DR values significantly different from chance (DR=0.5) level, indicating preferential responding into the CS+ reinforced nose poke port. Similarly, Ser 845 mutant mice showed preferential responding for the CS+, and their DR values did not differ from those of their WT controls (t(14)=0.41, p = 0.69). Analysis of DR values for double phosphorylation site mutant mice suggested that these mice showed a small preference for the CS+ reinforced nose poke port as well, although this difference only approached significance. Additionally, DR values for these mice were significantly attenuated relative to their WT controls (t(11)=2.7, p < 0.05). Finally, Ser 831 mice failed to show CS+ discriminated responding altogether (i.e., no significant difference from 0.5), and their DR values were significantly lower than those of their WT controls (t(21)=2.58, p (0.05)).
Table 1.
Discrimination ratios calculated for the test results for conditioned reinforcement in GluR1 phosphorylation mutant mice.
| Different from chance (0.5)* |
||||
|---|---|---|---|---|
| Group | Genotype | Ratio | T-value | p-value |
| Double | WT | 0.87±0.03 | 12.4 | <0.0001 |
| KO | 0.66±0.06 | 2.42 | =0.07 | |
| Ser 845 | WT | 0.88±0.04 | 8.63 | <0.0001 |
| KO | 0.89±0.01 | 26.8 | <0.0001 | |
| Ser831 | WT | 0.78 ± 0.03 | 11.83 | <0.0001 |
| KO | 0.6 ±0.06 | 1.65 | = 0.23 | |
Based on one-sample t-test to determine statistical difference from a hypothesized value of 0.5.
Finally, Figure 3 shows the time course of nose-poke responding of WT and the three different GluR1 phosphorylation site mutants during the test for conditioned reinforcement. Inspection of these data reveals that the deficit in discriminated responding for the conditioned reinforcer in the double phosphorylation and single Ser 831 phosphorylation site mutants was evident at the start of the session and not due to differences in extinction rate as a function of the mutation. Consistent with the analyses of the total response data and discrimination ratio’s, three-way ANOVAs of these data yielded only significant main effects of Stimulus (Fs > 20.7, ps < 0.001), Genotype (Fs > 4.6, ps < 0.05) and interactions between Stimulus and Genotype (Fs > 8.7, ps < 0.05) for both double and Ser 831 mutant groups. By contrast, in the Ser 845 condition, only the main effect of Stimulus reached significance (F(1,14)=57.9, P < 0.001) and no main or interaction effects of Genotype were seen.
Figure 3. Time course of nose-poke responding during the test for conditioned reinforcement in GluR1 phosphorylation site mutants.
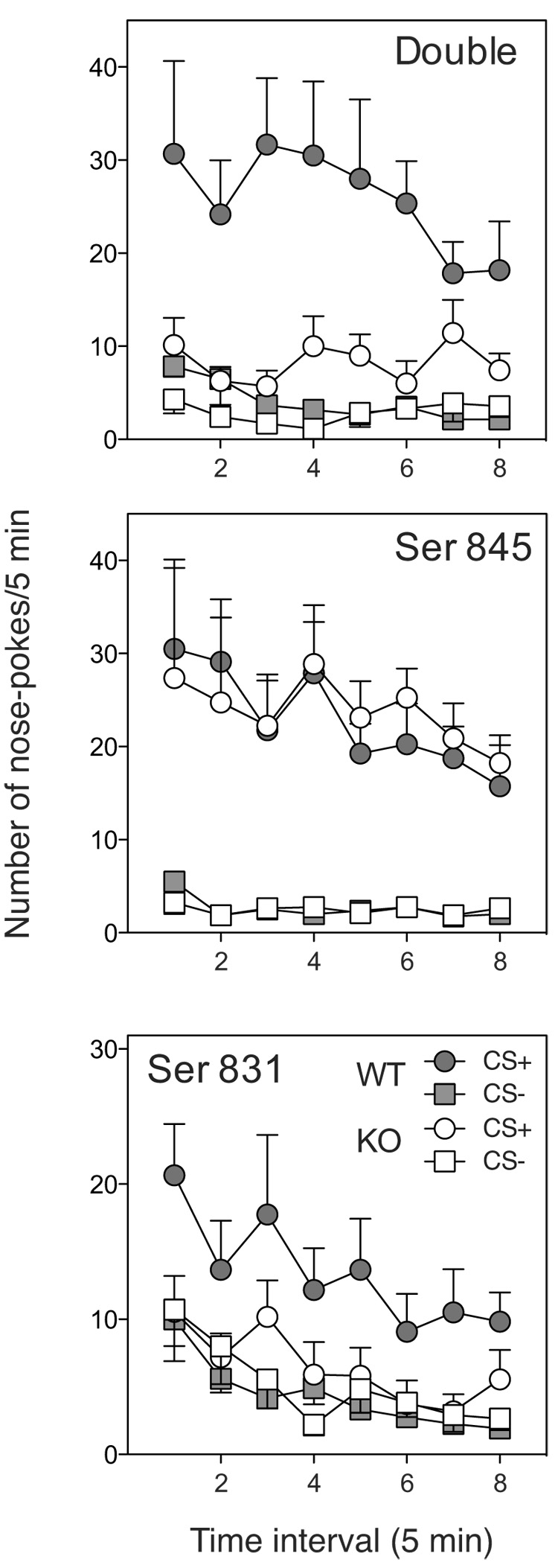
Average number of lever presses/5 min bin during the test for conditioned reinforcement in double and single phosphorylation mutant and WT mice. The error bars show ± SEM.
Taken together, the results from the conditioned reinforcement test sessions suggest that AMPA GluR1 phosphorylation plays a necessary role in the ability of environmental cues to become endowed with reinforcing value and that this role is specific to Serine 831 phosphorylation site of the AMPA GluR1 receptor.
Importantly, the deficits seen in conditioned reinforcement were specific to responding on the nose-poke port producing the CS+. In all three cases, WT and mutant mice had comparable baseline (CS−) responses on the measure (nose poke) used to assess incentive learning (Fig 2. b, d, f; effects of Stimulus provided above). Additionally, figure 4 shows that the phosphorylation mutations did not interfere with normal food cup responses (also a nose-poke; bottom) or overall locomotor activity levels (top) during the test session for conditioned reinforcement (Fs < 0.81, Ps > 0.3). These results are consistent with a selective Ser 831-dependent deficit in incentive learning and suggest that generalized performance deficits, impaired mobility or motivational state are unlikely to have accounted for the deficits in conditioned reinforcement.
Figure 4. Activity and food cup entries during the test sessions for conditioned reinforcement.
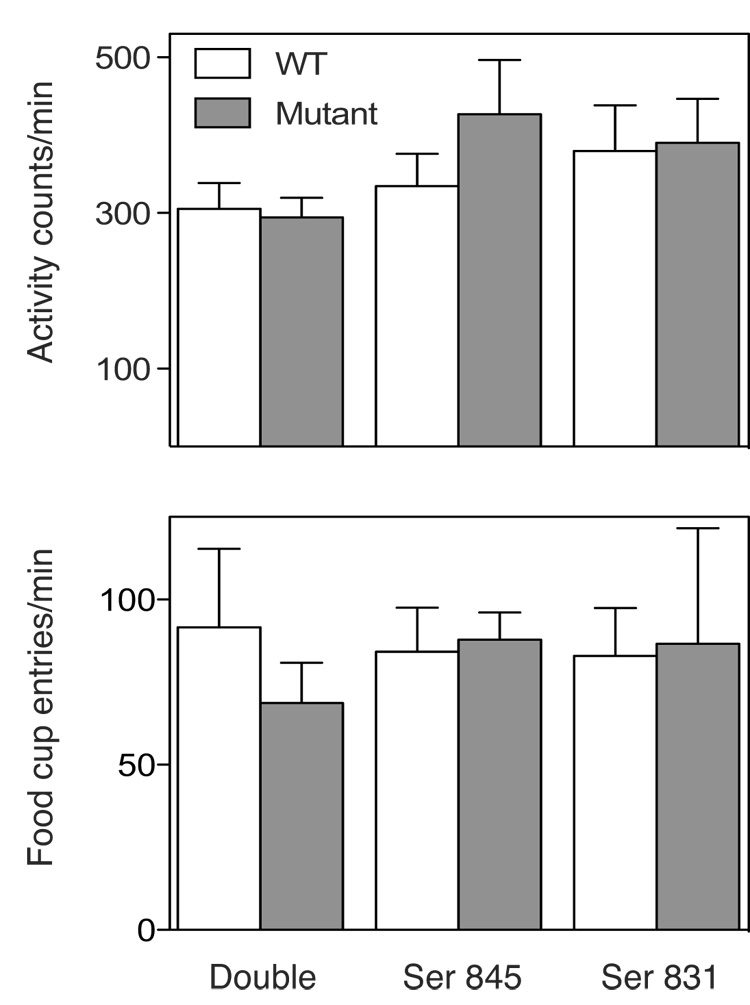
Average number of locomotor activity counts (panel a) and food cup entries (panel b) during the tests for conditioned reinforcement of double and single point phosphorylation site mutant and WT mice. The error bars show ± SEM.
Primary (food) reinforcement
To assess whether the deficits seen in double and Ser 831 phosphorylation site mutant were specific to the conditioned reinforcement task, the same phosphorylation mutant mice were tested for instrumental responding for liquid-food reward on two consecutive test sessions. As shown in Figure 5, unlike responding for the conditioned reinforcer (Pavlovian CS+), food-reinforced instrumental responding was unaffected by the phosphorylation mutations. Two-way ANOVA on responding on the reinforcement lever revealed no significant effects of Session (Fs < 4.2, ps > 0.06), Genotype (Fs < 0.4, ps > 0.5) or Session × Genotype (Fs < 0.49, Ps > 0.5) indicating that instrumental response levels reinforced by a primary (i.e., non-conditioned) reinforcer did not differ between the phosphorylation mutants and WT mice.
Figure 5. Instrumental responding for liquid food reward.
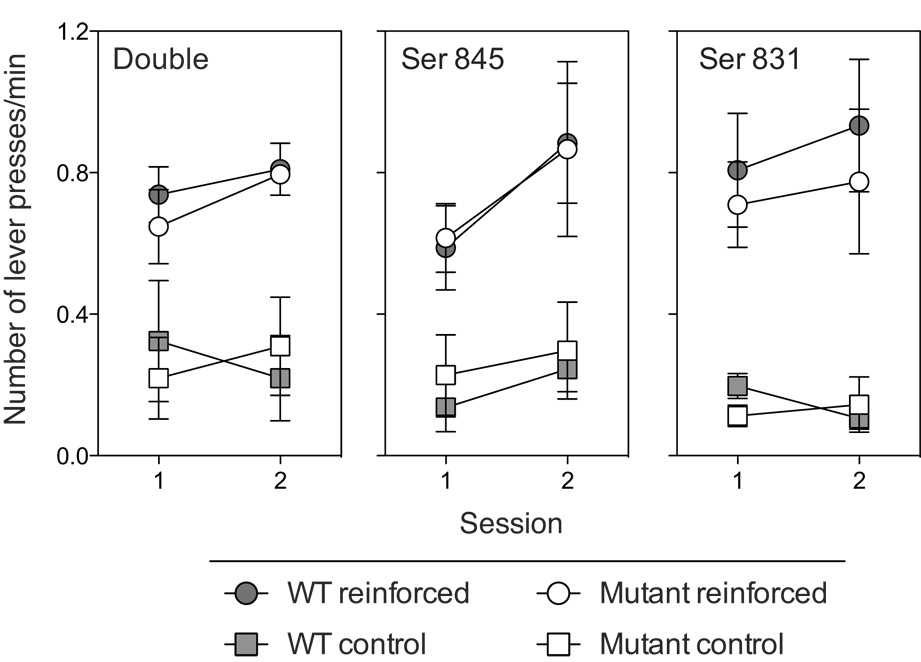
Average number of lever presses on the food reinforced or non-reinforced (control) levers during two consecutive training sessions in double and single point phosphorylation site mutant and WT mice. The error bars show ± SEM.
Discussion
Our results provide evidence for a learning phenotype in mice with a constitutive genetic mutation of a specific phosphorylation site on the AMPA-GluR1 receptor. In contrast to mice with targeted mutations of the AMPA GluR1 Ser 845 phosphorylation site – which showed no obvious deficits on any of our behavioral measures – selective mutations of the Ser 831 site (or both phosphorylation sites), resulted in a profound deficit in responding that earned the Pavlovian conditioned reinforcer. This deficit appeared specific to conditioned reinforcement: overall activity levels, magazine entries and instrumental nose-pokes to CS− did not vary as a function of genotype. Additionally, when these mice were tested in a separate experiment for responding for a primary reward, instrumental response rates were unaffected by the mutations - although we note that there were a number of procedural differences between the two experiments including the operant manipulandum (nose-poke versus lever press) used to assess operant response rates, and the presence versus absence of liquid reward in testing. Nonetheless, these results provide the first demonstration of an impairment that implicates Ser 831 phosphorylation of the AMPA GluR1 receptor as a critical mechanism for a form of incentive learning in which neutral environmental cues become endowed with the properties to serve as goals themselves.
In the present study we used a behavioral procedure of incentive learning that has been well characterized in rats and monkeys and which we have adapted to produce a robust measure of conditioned reinforcement in mice [11]. The neural circuitry thought to underlie conditioned reinforcement effects has been well characterized. For instance, lesion experiments in rats have revealed that the forebrain connections of the amygdala complex with the ventral striatum and prefrontal cortex provide essential neural circuitry for this type of incentive learning [e.g., 12]. Only recently have transgenic mouse models been used in an attempt to explore the molecular basis of conditioned reinforcement and related forms of incentive learning [e.g., 9, 13, 14, 15]. For instance, in a particularly elegant set of studies, Mead and Stephens [8, 16] used AMPA GluR1 and GluR2 subunit knockout mice and reported that whereas the AMPA-GluR1 receptor is critical for conditioned reinforcement (and second-order instrumental responding), the AMPA-GluR2 receptor was not (but was critical for single-reinforcer Pavlovian-instrumental transfer). The current results, therefore, are consistent with these findings, and provide an important extension by identifying a critical regulatory molecular mechanism in the Ser 831 phosphorylation site.
In an earlier study, Lee et al [6] reported that, compared to WT littermates, double Ser 845 + 831 GluR1 phosphorylation site mutant mice essentially lack NMDA receptor-dependent LTD and show reduced LTP in CA1 region. Additionally, although showing normal acquisition on a spatial learning and memory task (Morris water maze), these mice were unable to retain this memory after 8–24 hr. This neural plasticity in hippocampus, however, critically depends on Ser 845, and not Ser 831 phosphorylation; mice with the Ser 831 knock-in mutation display normal plasticity at CA1 synapses (LTP and LTD) (unpublished findings). It would appear therefore that, depending on the learning and memory task (e.g., Morris water maze spatial memory versus conditioning reinforcement) and/or underlying brain substrate (e.g., hippocampus versus BLA), different molecular (i.e., phosphorylation) mechanisms are engaged to regulate AMPA-GluR1 dependent synaptic plasticity. This hypothesis remains to be explored but the current findings suggest that future studies into the role of AMPA receptor phoshorylation mechanisms in regions others than the hippocampus, in particular the BLA, are likely to yield some unique findings.
Whilst keeping this in mind, in CA1 region of the hippocampus the functional role of the Ser 845 versus Ser 831 phosphorylation sites on AMPA GluR1 receptor has been extensively characterized and is complex. Under normal conditions, Ser 831 is phosphorylated by Ca(2+)/Calmodulin-dependent protein kinase II (CamKII) or Protein Kinase C (PKC), and the Ser 845 site is phosphorylated by Protein Kinase A (PKA) [4, 5]. Phosphorylation of both these sites on the GluR1 subunit of AMPA receptors changes with LTP and LTD induction such that LTP is associated with increased phosphorylation and LTD is correlated dephosphorylation of GluR1 [e.g., 4, 17]. However, the specific phosphorylation site modulated during LTP and LTD is dependent on the history of synaptic plasticity. In naïve synapses, LTP induction increases phosphorylation on Ser 831 while in previously depressed synapses LTP induction increases phosphorylation on Ser 845 [4, 17]. In turn, LTD induction in naïve synapses results in the dephosphorylation of Ser 845, while LTD induction in previously potentiated synapses leads to the dephosphorylation of Ser 831 [17].
Of course, without additional experiments, it would be highly speculative at this time to hypothesize as to the cellular mechanisms by which the absence of Ser 831 could have modulated the type incentive learning reported here. However, it is well know that GluR1 is critical in different forms of synaptic changes in many regions including in the amygdala. For instance, Rumpel et al [7] recently reported that, in the lateral amygdala, cued-fear conditioning was closely associated with synaptic incorporation of GluR1 receptors. Also, gria1 mutant mice show deficits on behavioral tasks of acquired affect and motivation that are known to depend on normal function in neural circuitry that includes the basolateral amygdala (BLA), as well as the ventral striatum, and prefrontal cortex [e.g., 8]. It seems reasonable, therefore, to speculate that the Ser 831 deletion modulated incentive learning in our task by interfering with the establishment of synaptic plasticity in the basolateral amygdala, for instance by interfering with the ability of CamKII activity to alter GluR1 conductance and/or produce normal incorporation of AMPA receptors into the post-synaptic cell surface.
In summary, we used a behavioral model of incentive learning that has been well characterized and that is known to involve forebrain circuitry that includes the BLA, ventral striatum, and prefrontal cortex. The current findings provide new evidence for a critical molecular mechanism that involves phosphorylation of AMPA-GluR1 receptors by Ca(2+)/Calmodulin-dependent protein kinase II (CaMKII) or Protein Kinase C (PKC) within this network that underlies this specific form of learning. Of course, future experiments, aimed at directly targeting GluR1 phosphorylation processes within this circuitry using, for example, viral-mediated gene knockdown or phosphorylation site- or kinase-specific inhibitors, will be required to provide direct support for this hypothesis. Such explorations will be well worth it as Pavlovian conditioning mechanisms are thought to importantly contribute to the regulation of ‘normal’ emotional and motivated behavior, as well as maladaptive conditions, such as eating disorders and drug addiction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25(11):578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 2.Zamanillo D, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284(5421):1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 3.Reisel D, et al. Spatial memory dissociations in mice lacking GluR1. Nat Neurosci. 2002;5(9):868–873. doi: 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- 4.Barria A, et al. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276(5321):2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 5.Roche KW, et al. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16(6):1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 6.Lee HK, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112(5):631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 7.Rumpel S, et al. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308(5718):83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 8.Mead AN, Stephens DN. Selective Disruption of Stimulus-Reward Learning in Glutamate Receptor gria1 Knock-Out Mice. J Neurosci. 2003;23(3):1041–1048. doi: 10.1523/JNEUROSCI.23-03-01041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson AW, et al. Impaired outcome-specific devaluation of instrumental responding in mice with a targeted deletion of the AMPA receptor glutamate receptor 1 subunit. J Neurosci. 2005;25(9):2359–2365. doi: 10.1523/JNEUROSCI.4146-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy A, et al. Dissecting the role of N-myc in development using a single targeting vector to generate a series of alleles. Curr Biol. 1998;8(11):661–664. doi: 10.1016/s0960-9822(98)70254-4. [DOI] [PubMed] [Google Scholar]
- 11.Crombag HS, Galarce EM, Holland PC. Pavlovian influences on goal-directed behavior in mice: The role of cue-reinforcer relations. Learning & memory. 2008 doi: 10.1101/lm.762508. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 13.Yin HH, Zhuang X, Balleine BW. Instrumental learning in hyperdopaminergic mice. Neurobiol Learn Mem. 2006;85(3):283–288. doi: 10.1016/j.nlm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Wiltgen BJ, et al. The influence of Pavlovian cues on instrumental performance is mediated by CaMKII activity in the striatum. Eur J Neurosci. 2007;25(8):2491–2497. doi: 10.1111/j.1460-9568.2007.05487.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnson AW, et al. A selective role for neuronal activity regulated pentraxin in the processing of sensory-specific incentive value. J Neurosci. 2007;27(49):13430–13435. doi: 10.1523/JNEUROSCI.4320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mead AN, Stephens DN. Involvement of AMPA receptor GluR2 subunits in stimulus-reward learning: evidence from glutamate receptor gria2 knock-out mice. J Neurosci. 2003;23(29):9500–9507. doi: 10.1523/JNEUROSCI.23-29-09500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HK, et al. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405(6789):955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]


