Abstract
In infant rats, low doses of ethanol have been found to attenuate the aversive representation of an unconditioned stimulus (US) as assessed through a revaluation paradigm. This may be explained by early anxiolytic properties of EtOH. The present set of experiments was aimed at analyzing possible mechanisms of these putative anti-anxiety effects of EtOH. In a the first experiment, EtOH's effects upon the expression of citric acid-induced distress calls were compared with varying doses of midazolam (MDZ), a fast-acting GABAA agonist. Similar calming effects of 0.5 g/kg EtOH and 0.09 mg/kg MDZ were observed. Both drugs were then assessed in their capability to alter the expression of a conditioned aversion by devaluing the US. Aversive conditioning was conducted on postnatal day 14 (PD14) by pairing a lemon odor (conditioned stimulus, CS) with intraoral stimulation of citric acid (US). Control animals experienced both stimuli in an explicitly unrelated fashion. On PD 15 pups were briefly exposed to the citric acid solution under the effects of 0.5 g/kg EtOH, 0.09 mg/kg MDZ, or the respective vehicle for each drug. Pups were then tested in a two-way odor preference test (lemon vs. cineole). Both vehicle and MDZ-treated animals spent significantly less time near the lemon CS, thus expressing a citric-acid mediated odor aversion. This conditioned response was completely inhibited in pups that received 0.5 g/kg EtOH. Locomotor patterns at test were not affected by either EtOH or MDZ administration. A higher dose of MDZ (0.18 mg/kg, i.p) was also ineffective in attenuating the aversive memory. In summary, EtOH's devaluating capabilities are not shared by MDZ, indicating that these effects of EtOH may not be GABA-mediated. Appetitive motivational properties of EtOH or non-GABAA-mediated anti-anxiety effects (i.e, NMDA-related) could underlie this devaluation effect of ethanol.
Keywords: aversive conditioning, devaluation, anxiolytic, ethanol, midazolam, infant
Introduction
Ethanol (EtOH) possesses motivational properties likely to affect approach and consumption of the drug. Low to moderate EtOH doses can serve as an appetitive stimulus, causing animals to prefer discrete tactile stimuli signaling its effects (Bechtholt and Cunningham, 2005; Philpot et al, 2003). On the other hand, aversive effects of the drug are easily found by pairing a novel flavor or distinctive place with the post absorptive effects of EtOH. Animals will later exhibit strong taste or place avoidance, particularly when a high dose of EtOH is employed (Broadbent et al., 2002; Fidler et al., 2004; Pautassi et al., 2002).
EtOH's motivational properties are not restricted to its appetitive and aversive features. The drug also exerts anti-anxiety effects similar to those found in clinically proven anxiolytic drugs. Wilson and coworkers (2004) found that both EtOH and the GABA-A agonist diazepam caused dose-dependent increases in time spent in the open arms of an elevated plus maze. Both drugs also reduced burying behavior in the prod-burying task (Wilson et. al., 2004). These negative reinforcing properties of ethanol appear to play an important role in modulating patterns of ethanol use and abuse (Ahmed et al., 2002; Koob et al., 2004).
Considerable effort has been devoted to the analysis of EtOH's appetitive and aversive properties (Cunningham et al., 2000). Yet, there is a relative scarcity of research related to EtOH's anti-anxiety effects, particularly early in ontogeny. It is still uncertain whether rat pups perceive EtOH's anxiolytic properties and, if so, what mechanisms underlie this phenomenon. This void in the literature can be explained by a lack of appropriate age-related tests. Most of the techniques that are employed in adult rodents are not suitable when focusing on younger animals. For instance, standard screening tests for anti-anxiety effects, such as the elevated plus maze (Olivier et al., 1994) or the light-dark transition task (Bourin and Hascoet, 2003), are precluded in the infant due to obvious sensory limitations. Given these limitations, the measurement of ultrasonic vocalizations (USVs) in socially isolated infant rats has provided a useful and sensitive test for anxiolytic effects in early infancy. Rat pups emit USVs when placed in isolation from their dam and littermates (Kraebel et al., 2002) or when exposed to stressors such as low temperature (Blumberg and Alberts, 1990). These responses have been described as distress calls related to crying observed in other mammal species (Wilson and Insel, 1991) and are sensitive to several pharmacological manipulations. Anxiogenic agents enhance USVs (Branchi et al., 2001), whereas drugs that facilitate GABAergic neurotransmission, such as midazolam, attenuate their emission (Dirks et al., 2002). Engel and Hard (1987) observed that USVs induced by maternal separation were dose-dependently reduced by low-dose ethanol (0.5 g/kg) as well as by diazepam. The calming effects of the latter drug were reverted by Ro 15-1788 (a benzodiazepine-receptor-antagonist). However, only picrotoxin (a GABAergic antagonist) reversed the effects of EtOH on stress-induced USV production. These results suggest that EtOH may exert anxiolytic properties similar to those of diazepam although the mechanisms underlying these effects might be different from those exerted by benzodiazepines.
The separation-induced USVs model takes advantage of an innate, unlearned measure of anxiety. These types of paradigms are known as unconditioned models of anxiety. On the other hand, conditioning techniques encompass procedures in which animals are exposed to an initially neutral cue (conditioned stimulus, CS) while experiencing an anxiogenic and/or aversive event (Hitzemann, 2000). Subsequently, levels of anxiety are measured in terms of the animal's behavioral response to presentation of the CS alone. For example, in the contextual fear paradigm, nociceptive stimulation (footshock, US) is associated with a complex environmental representation (context, CS). When re-exposed to the CS, animals display several conditioned responses (CRs), including freezing (Bustos et al., 2006). Aversive memories acquired through pairings of a CS and an US are susceptible to modification after initial acquisition. Further pairings comprising the original US and alternative USs characterized by either similar or opposite affective values result in an enhanced (i.e., inflated) or reduced (i.e., devalued) conditioned response, respectively.
A devaluation paradigm was recently employed by Pautassi et al. (2006) to test anxiolytic effects of EtOH in young rats. Pups were stimulated (conditioning phase) with a salient odor (lemon scent, CS) while intraorally infused with an aversive tastant (citric acid, US). Twenty-four hours later these animals were briefly re-exposed to the acid tastant while intoxicated with varying doses of ethanol or with vehicle (revaluation phase). When tested in a two-way odor preference test, vehicle treated animals spent significantly less time near the lemon scent than their control counterparts. Thus, these animals expressed a citric acid-mediated conditioned avoidance. This learned response was significantly ameliorated in those animals that had experienced the post-absorptive consequences of low ethanol doses (0.25 – 1.25 g/kg) during the devaluation phase.
These results indicate that ethanol's post-absorptive consequences were effective in reducing the aversive value of an innately aversive stimulus. In other words, experiencing citric acid under the effects of ethanol rendered the original US (citric acid) less aversive, which in turn decreased the capability of the lemon CS to elicit escape or avoidance responses. These results may be interpreted in terms of EtOH exerting anxiolytic effects early in infancy. Since it is known that GABAA receptors are functional in infants (Bianchi et al., 2005), it could be hypothesized that these results are related to ethanol's activation of the GABA system. Nonetheless, the Pautassi et al. (2006) devaluation experiment does not preclude an alternative possibility. The devalued aversive CR may not have been the result of ethanol's anxiolytic effects, but rather were derived from inherent appetitive properties of the drug. Indeed, EtOH's appetitive properties have been observed in preweanlings (e.g., Molina et al., 2006b; Nizhnikov et al., 2006) and periadolescent rats (Fernandez-Vidal et al., 2004).
One aim of the present set of experiments was to analyze the capability of a low EtOH dose (0.5 g/kg) to modulate the expression of USVs in young animals. USVs were elicited not only by maternal separation (as in Engel and Hard, 1987) but also through explicit presentations of an innately aversive US (intraoral citric acid). Parameters of the sapid US (intensity, temporal duration and schedule of stimulation) replicated those that had been employed in Pautassi's et al. (2006) devaluation procedure. Ethanol's effects upon USVs were also compared with varying doses of midazolam (MDZ). The explicit intention was to equate ethanol and an alternative GABAA agonist in terms of their effects in an unconditioned model of anxiety. After this was accomplished, the Pautassi et al. (2006) devaluation model was recreated. Subsequent experiments compared the effectiveness of EtOH and MDZ in ameliorating the expression of a conditioned aversion. The first step was to establish doses that could equate MDZ and EtOH in terms of the rat's reactivity to an aversive US. Then, the effects of these drugs on the representation of an aversive memory were tested. The purpose of these procedures was to test the possibility that ethanol's effects in the devaluation task (Pautassi et al., 2006) are mediated by a GABA-A -related anxiolytic effect. If this hypothesis is correct, post-conditioning administration of EtOH and MDZ should cause similar inhibitory effects upon the expression of a conditioned aversive memory. Alternative findings would suggest that (a) other neural systems (e.g., NDMA receptors) mediate these proposed early anxiolytic effects of EtOH or (b) that rather than anti-anxiety effects, alternative, perhaps appetitive effects of the drug could be responsible for counteracting the aversive learning under analysis.
General Methods
Subjects
Sprague-Dawley rat pups (14 days of age at the start of the Experiments, weight: 27-40 g) born and reared at the Center for Developmental Psychobiology (Binghamton University, USA) were employed. For breeding, 1 male and 1 female (Taconic, Germantown, NY) were housed together in a wire mesh-hanging cage. The paper tray under the cage was checked daily for plugs and the day a plug was found was considered embryonic day zero (E0). Upon discovery of the plug the female was removed from the cage and housed with another pregnant female in a standard plastic maternity cage until E19, when they were separated and placed into individual cages. Animals were housed in a temperature-controlled (22°C) vivarium maintained on a 12-hr light / dark cycle (lights on at 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. Births were examined daily and the day of birth was considered as PD0. On PD1 litters were culled to 10 pups, keeping sex ratios equal whenever possible. Pups remained with the dam until PD14, when they were removed for the experiments. Rats used in these experiments were maintained and treated in accordance with the Guide for Care and Use of Laboratory Animals (NIH, Institute of Laboratory Animal Resources, 1996). The Binghamton University Institutional Animal Care and Use Committee approved all of the procedures used in this study.
Cannulation Procedures
Experiments conducted in this study required animals to be intraorally implanted with polyethylene tubing cannulae. These devices were made from 6-cm sections of PE 10 polyethylene tubing (Clay-Adams). A small flange was created in one end of these devices. The unflanged end was attached to a curved 27-G ½ precision glide needle (Becton Dickinson and Co., Rutheford, N.J). The needle was pulled through the medial internal surface of the cheek of the subject. Consequently, the flanged end of the cannulae rested over the oral mucosae while the remainder exited from the mouth. Anesthesia is not needed to conduct this procedure, which requires approximately 7 seconds per animal. Location of the cheek cannulae did not cause any interference with later EtOH intragastric intubations, even when cannulation was conducted across days (Exp. 2 a, b). In the latter case, alternate cheeks (i.e, right and left) were cannulated each day. Cannulae were taken out by gently pushing the unflanged end of the device until all tubing was removed of the oral cavity, a procedure that took no more than 7 seconds per animal. It has been shown that cannulation procedures cause minimal levels of stress in preweanlings (Spear et al., 1989).
EtOH and midazolam administration procedures
EtOH intragastric administration was conducted by gently introducing an 8-cm section of PE 10 polyethylene tubing (Clay-Adams) into the pup's oral cavity. The tubing was connected to a 1cc tuberculin syringe mounted with a 27 G ½ needle (Becton Dickinson and Co., Rutheford, N.J). About 4 cm of tubing were gently guided into the subjects' stomach prior to the actual delivery of EtOH. Midazolam was administered via IP injection. Intraperitoneal injections took less than 10 seconds and were performed in a region situated approximately between the diaphragm and the genitalia.
The EtOH dose (0.5 g/ kg) was achieved by administering 0.017 ml/g of a 3.7 % v/v ethanol solution (190-proof Ethanol, Pharmaco, Brookfield; vehicle: distilled water). Midazolam doses were derived from two main stocks. The highest dose (1.5 mg/kg) was derived from a 0.3 mg/ml solution and the remaining drug doses (0.09, 0.18, 0.37 or 0.75 mg/kg) from a 0.075 mg/ml solution (physiological saline being the vehicle). This was done in order to keep volume of midazolam injected similar across doses. Distilled water and saline (0.89 %, v/v) were employed as vehicles for the ethanol and midazolam solutions, respectively.
Statistical Procedures
Preliminary analysis of the ultrasonic vocalization data consistently showed no main significant effects of gender or interaction with other factors. Similarly sex was never found to interact with drug treatment or conditioning in the odor preference test (Exp. 2a, b). Hence, descriptive and inferential analysis of the data has been performed by collapsing across gender. Fixed-factor Analysis of Variance (ANOVA), that eventually included a repeated measure factor, was performed. Fisher's least significant difference (LSD) post-hoc tests were used in order to further analyze significant main effects or to determine the loci of significant interactions comprising between-group factors. In order to avoid spurious positives, the alpha value for the LSD tests was lowered by employment of the Bonferroni correction to account for the number of comparisons being performed (Abdi et al., 2007). When necessary, planned comparison contrasts were conducted. Probability of type I error for ANOVA main effects and interactions as well as for planned contrasts was set at 0.05.
Experiment 1
Recent studies (Pautassi et al., 2005; 2006) support the notion that ethanol's negative reinforcing (anti-anxiety-like) properties modulate the acquisition of aversive memories and/or the retrieval of their hedonic contents in infant rats. Anxiolytic attributes of ethanol are dependent, at least partially, on GABAergic mechanisms (Koob, 2004). Midazolam (MDZ) has been observed to disrupt fear expression or fear reconsolidation processes, to attenuate conditioned taste aversions and mitigate stress-like responses (Bustos et al., 2006; Nobre and Brandao, 2004; Yasoshima and Yamamoto, 2005). These effects have been reported during adulthood. However, little is known about infantile sensitivity to this GABAergic agent (as an anticonvulsant agent, see Kubova and Mares, 1992).
The goal of this experiment was to analyze and equate possible calming effects of ethanol and MDZ upon infantile USVs elicited by oral citric acid infusions and/or social isolation. This experiment was conceived as a first step leading towards the understanding of mechanisms likely to modulate negative reinforcing effects of ethanol in the preweanling rat. Results obtained from Experiment 1 will be employed as a springboard to further analyze early anxiolytic effects of ethanol by means of a devaluation technique, as used by Pautassi et al. (2006). For this reason, parameters related to drug dose, US intensity, schedule of US infusion, and developmental stage of the animal were similar to those employed by Pautassi et al. (2006).
Material and Methods
Experimental Design
The design was a 9 (drug treatment) × 2 (assessment phase) mixed factorial. Experimental subjects were divided into 9 groups defined by drug treatment [untreated, vehicle i.p, vehicle i.g, ethanol (0.5 g/kg i.g), and midazolam (0.09, 0.18, 0.37, 0.75, or 1.5 mg/kg]. The testing procedure was further divided into 2 phases. Phase one tested response to social isolation following drug treatment while phase two tested response to social isolation and infusion of a 0.2% v/v citric acid solution known to be highly aversive to rats of this age (Molina et al., 1996; Scalera 2004). To eliminate confounding of litter with treatment effects, no more than one subject from a given litter was assigned to the same treatment condition (Holson and Pearce, 1992). Each condition included an equal number of male and female subjects. The dependent variable examined was number of USVs emitted during the 2 phases of testing.
USV-Testing Procedure
Seventy-four experimental subjects, representative of nine litters, were removed from their home cage on PD 14 and isolated in a plastic breeding tub with dividers set into place for 30 minutes. This tub was kept at 34° C by means of a heating pad placed underneath. Following this initial isolation period subjects were implanted in their cheek with a polyethylene cannula (Pautassi et al., 2002).
After cannulation, pups were placed back into the holding tub for 30 minutes. They were then exposed to one of the 9 drug treatments outlined above [untreated, vehicle i.p or i.g, ethanol (0.5 g/kg i.g), or midazolam (0.09, 0.18, 0.37, 0.75, or 1.5 mg/kg)]. The number of subjects assigned to each treatment condition was 8-9 pups.
Following drug treatment they were placed back into the holding tub for 5 minutes. Untreated animals were handled the same as all other pups but were not injected or intubated. Following the 5-minute period pups were placed individually into a sound-attenuated chamber (Med Associates, St Albans, VT) with cotton lining the floor. USV's were recorded using an Avisoft Ultrasoundgate 416 recorder (Berlin, Germany) for 10 minutes. This initial period was defined as phase 1 (social isolation) of the experiment. Subsequently, they were intraorally stimulated with citric acid (phase 2, social isolation + citric acid infusion) using a Harvard Apparatus Syringe Pump (Natick, MA). Specifically, pups received 4 citric acid pulses every 60 seconds (0.2% w/v, rate of infusion: 0.9 ml/min, 3 s on, 12 s off) for 10 minutes. Vocalizations in the range of 35 – 70 kHz were recorded in a minute-by-minute basis for both phases of the experiment. The sequence of presentation of stressors (i.e. social isolation followed by social isolation plus citric acid infusions) was chosen so as to obtain a baseline-like level of USV responding prior to adding of a distinctive US (citric acid). We also tried to avoid a possible negative contrast effect (Mitchell and Flaherty, 1998) that might have taken place if a potentially greater stressor (citric acid infusions) was followed by a theoretically lesser stressor such as social isolation. USVs were later analyzed (Avisoft Ultrasoundgate Software, Berlin, Germany) to generate total number of USVs per bin of evaluation (2 minutes per bin).
Data Analysis
Ultrasonic vocalizations were analyzed using a 3 way repeated measures ANOVA [drug treatment (0.5 g/kg EtOH, 0.09 mg/kg MDZ, 0.18 mg/kg MDZ, 0.37 mg/kg MDZ, 0.75 mg/kg MDZ, 1.5 mg/kg MDZ, vehicle i.p, vehicle i.g and untreated controls, UT) × phase (1 or 2) × bin of evaluation (5 bins of two minutes each: repeated measures factor)].
3.2. Results
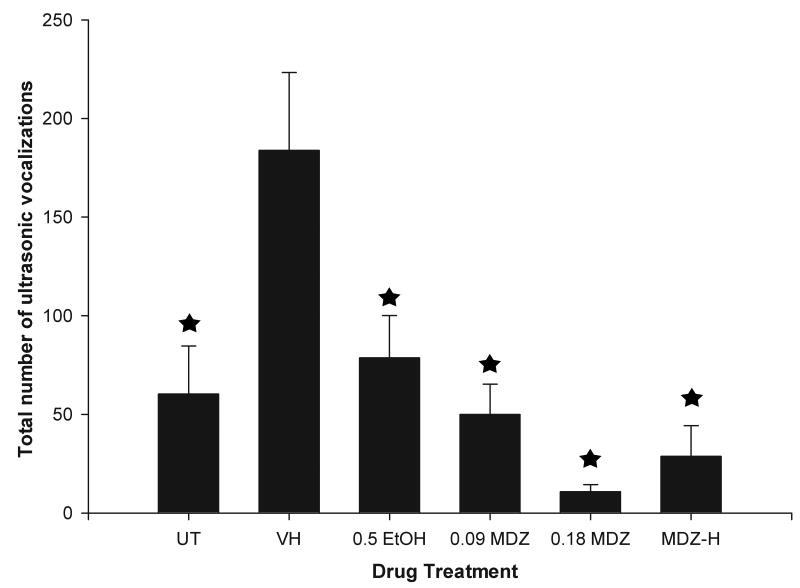
The ANOVA indicated a main significant effect of drug treatment upon USV expression [F(8, 65) = 4.32; p < 0.0005]. Subsequent planned contrasts between groups indicated that vehicle-treated animals had greater USV production than untreated controls, [F(1, 65) = 8.52; p < 0.005]. In turn, pups administered 0.5 g/kg EtOH or 0.09 mg/kg MDZ did not differ in their USV emission, [F(1, 65) = 0.34; p = 0.55]. These groups exhibited similar number of vocalizations when compared with untreated subjects, [F(1, 65) = 0.009; p < 0.92]. Administration of the highest MDZ doses was associated with even lower USV emission. No significant main effects of bin or phase were found. Also, no significant interactions involving bin, treatment or phase were detected by the ANOVA. Results are depicted in Figure 1.
Figure 1.
Total number of ultrasonic vocalizations (USVs) exhibited by pups in Experiment 1 as a function of drug treatment [UT (untreated animals), VH (vehicle treated animals), 0.5 g/kg ethanol, 0.09 or 0.18 mg/kg midazolam or pups treated with higher doses of midazolam, MDZ-H]. In order to facilitate data visualization, groups treated with midazolam doses eqivalent to 0.37-1.5 mg/kg which did not differ in their levels of USV emissions have been collapsed in a sole condition (MDZ-H group). VH group refers to animals administered with 0.0 g/kg ethanol or 0.0 mg/kg midazolam. These groups had similar levels of USV emission and have also been collapsed. Asterisks indicate significant differences from the VH group (p < 0.05).
These results indicate that vehicle treated subjects increase their distress calling compared to untreated controls. Therefore, the mere act of handling and administering vehicle facilitated stress-mediated responding. In this context, EtOH had a protective effect on stress-induced vocalizations. Stimulation of GABA via a 0.09 mg/kg dose of MDZ, prior to stress, reduced the USV emissions when compared to vehicle treated subjects in a similar fashion to 0.5 g/kg ethanol. A MDZ dose-response effect was observed, with MDZ-H pups emitting almost no ultrasonic vocalizations.
Experiment 2
Experiment 2 a
In the previous experiment, ethanol and MDZ were assessed in terms of their influence on an unconditioned measure of anxiety, ultrasonic vocalization elicited by isolation and intraoral infusion of an aversive tastant. Interestingly, 0.09 mg/kg MDZ and 0.5 g/kg EtOH exerted similar ameliorating effects upon USV emission. As mentioned, post-conditioning EtOH (0.5 g/kg) reduces the expression of aversive non-drug mediated conditioning, a result presumably caused by anti-anxiety-like effects of the drug (Pautassi et al, 2006). The present experiment was designed to (a) replicate previous results showing devaluation of an aversive memory by low-dose EtOH and (b) compare devaluation effects by EtOH and MDZ on expression of an aversive memory. It should be noted that the dosage of both drugs corresponded to those shown to have equivalent calming effects on USV expression in Experiment 1. The rationale underlying this experimental strategy was to dissect the mechanisms underlying the putative anti-anxiety effects of EtOH. If MDZ effects are comparable to those of EtOH, not only would this result suggest that ethanol is exerting anti-anxiety effects in the young but also that a GABAergic system could be mediating this phenomenon.
Methods
Experimental design
A 2 (conditioning) × 4 (drug treatment) experimental design was employed. Animals were placed in a lemon-scented chamber and intraorally infused with citric acid (Paired Groups: P) or they were given the citric acid infusion 2 hours prior to being placed into a chamber scented with lemon (Unpaired Groups: UP). The second independent factor was drug treatment administered to the pups during the devaluation phase. Subjects were given EtOH (0.5, g/kg, intragastric, i.g.), midazolam (MDZ, 0.09 mg/kg, intraperitoneal, i.p.) or the corresponding vehicle solutions for the preceding drugs, water or saline, respectively. Thus, this experimental design was composed of 8 groups. Number of animals in these groups was as follows: UP/EtOH, 12; UP/Water, 14, UP/MDZ, 11; UP/Saline, 11; P/EtOH, 14; P/Water, 16, P/MDZ, 12; P/Saline, 11.
Conditioning and Testing Procedures
The experimental procedure was divided into three phases.
First phase, conditioning (PD 14)
Pups (104 subjects, representative of thirteen litters) were removed from their maternal cages and placed, in couples, in holding chambers kept warm (32-34° C) by means of a heating pad underneath the holding cage. Whenever possible, these couples were composed by animals of the same sex. They were immediately weighed to the nearest 0.01 g and implanted with an intraoral cannula. Animals were left undisturbed in the holding chambers for two hours, until the start of the conditioning procedures. During each conditioning trial, animals were individually placed in Plexiglas boxes (27 × 11 × 15 cm). On one of the opposite walls a transparent Plexiglas receptacle (3 × 11× 15 cm) was placed. This receptacle contained a lemon-scented cotton square measuring 10 cm × 14 cm (1 ml of pure lemon oil, Lor Ann Oils Inc., Lansing, MI). Odor diffused into the conditioning box through 48 small holes (diameter: 0.5 cm) homogeneously distributed in the receptacle. Pups received intraoral infusions of a citric acid solution (0.2 % w/v; Sigma, St. Louis, MO) for 5 min, while in these chambers. The solution was delivered via an infusion pump (Harvard Apparatus syringe pump, Natick, MA) connected to the subject's cannula. The citric acid was delivered in a pulsating manner (3s on, 10 s off; infusion rate: 0.8 ml/min). Four of these 5-min conditioning trials were conducted. The duration of the interval between each trial (ITI) was 10 minutes. During the ITI pups were maintained in isolation in a heated cage located in a separate room. Control pups were first exposed to the taste US (citric acid) and 120 minutes later were placed in the lemon-scented odor chambers. These conditioning parameters were selected on the basis of previous studies showing that four pairings between citric acid and lemon-odor stimulation are optimal for generating reliable levels of odor-aversive conditioning (Pautassi et al, 2006). Pups were returned to their respective maternal chambers 60 minutes after the last conditioning trial. Cannulae were removed at the end of conditioning.
Second phase, devaluation (PD 15)
Pups were removed from the dam, individually implanted with an intraoral cannula on the opposite cheek relative to the one cannulated in the conditioning procedure and left undisturbed in heated holding cages. Two hours later animals were weighed and then administered with EtOH [0.0 (vehicle) or 0.5 g/kg, i.g.] or midazolam [0.0 (vehicle) or 0.09 mg/kg, i.p.]. Five minutes after administration, animals were placed in individual Plexiglas chambers (12 × 12 × 12 cm). Intraoral stimulation with citric acid was then conducted for 5 minutes (0.2 % v/v, 3 s on, 10 s off; infusion rate: 0.8 ml/min). After the end of this 5-min devaluation trial, pups had their cannula removed and were returned to their respective holding chambers for 120 minutes.
Third phase, odor preference test (PD 15)
Two hours after the devaluation procedure, pups were tested in terms of preference for lemon odor in a two-way odor location test. Testing was conducted in a room illuminated with a 40-watt red light. A clear Plexiglas rectangular chamber (26 × 16 × 11 cm) was employed as the testing apparatus. Each of the smaller opposite walls of this chamber had 36 holes. A Plexiglas receptacle was located in the external side of these walls. The receptacles contained either lemon or cineole (Sigma, Saint Louis) scented cotton (1 ml of either odorant). Testing started by gently placing the animal in the middle section of the apparatus. Time spent in the olfactory sections of the apparatus was recorded. Specifically, these sections (80% of the total apparatus) corresponded to the surfaces located either next to the lemon (40%) or the cineole (40%) scented cotton. The middle section (20%) of the apparatus was considered as a neutral area. Time spent on a given olfactory section was recorded whenever the head and the front paws were positioned over that section. Duration of locomotor activity (s) during test (defined as subject moving at least three paws) was also recorded. The dependent variables were registered in real time by an experimenter blind to the training conditions of the animals.
Data Analysis
Total time spent (s) over the lemon-scented section was the dependent variable under analysis. Since a neutral intermediate zone was taken into account with regard to odor preferences, exact reciprocal results were not expected if time spent over the alternative odor zone was analyzed. For this reason, odor preference scores were also analyzed in terms of the percentage of time spent over the lemon section of the test apparatus relative to time spent spent over the cineole side. Time spent over the neutral side was not taken into account for data analysis. As indicated by preliminary statistical analyses, all unpaired groups, independent of the pharmacological treatment received during the devaluation stage, showed similar amounts of absolute time spent over the lemon odor during the two-way odor preference test, F(3, 44) = 0.72; p = 0.55. Further analysis of the data based on percent time spent on the lemon section of the cage also indicated that these unpaired groups did not differ, F (3, 44) = 0.61, p = 0.61. Hence, these groups were collapsed into a unique experimental condition (UP). Absolute and percent time spent over lemon odor were analyzed by means of similar one-way ANOVAs, which considered treatment during conditioning (UP, P/EtOH 0.0, P/EtOH 0.5, P/MDZ 0.0 and P/MDZ 0.09) as independent between factors. Finally, locomotion scores were examined using a 2 × 4 ANOVA. Learning condition (paired or unpaired) and treatment at devaluation (EtOH 0.0 g/kg, EtOH 0.5 g/kg, MDZ 0.00 g/kg or MDZ 0.09 g/kg) served as between factors in this analysis.
Results
Unpaired animals spent approximately 200 s (75%) on the lemon-scented section of the test cage. Preference for the lemon CS was much less in paired pups that had been administered vehicle during the devaluation phase. As expected, these pups expressed a conditioned aversion response towards the lemon. Paired animals treated with MDZ displayed a similar avoidance profile. On the other hand, animals in the Paired/EtOH group exhibited odor preference patterns similar to those observed in UP control groups. Paired/EtOH pups had experienced the lemon odor in close contiguity with citric acid and were then re-exposed to the latter US while intoxicated with 0.5 g/kg EtOH. These results were supported by the inferential analysis. Specifically, treatment during conditioning exerted a significant main effect upon both absolute (s) and percent (%) time spent over the lemon-scented section of the testing apparatus, F (4, 96) = 17.87, F (4, 96) = 11.40; respectively, both p's < .0001. These effects were further analyzed by means of LSD's post- hoc tests. In both cases, they indicated that UP animals spent significantly more time over the lemon odor CS than either paired group subsequently treated with vehicle. Time spent near the lemon odor was also lower for animals in the P/MDZ 0.09 group than for UP animals. Paired pups that received ethanol during the devaluation phase, however, did not differ from unpaired control subjects in odor preference and had significantly greater preference for lemon odor than the remaining paired-vehicle groups. Absolute preference scores can be observed in Figure 2. Overall means and standard errors for percent time preference were as follows: Unpaired Controls = 73.38 ± 2.1, P/EtOH 0.0 = 53.16 ± 4.9, P/EtOH 0.5 = 68.05 ± 4.27, P/MDZ 0.0 = 49.00 ± 3.9, P/MDZ 0.09 = 50.50 ± 5.2.
Figure 2.
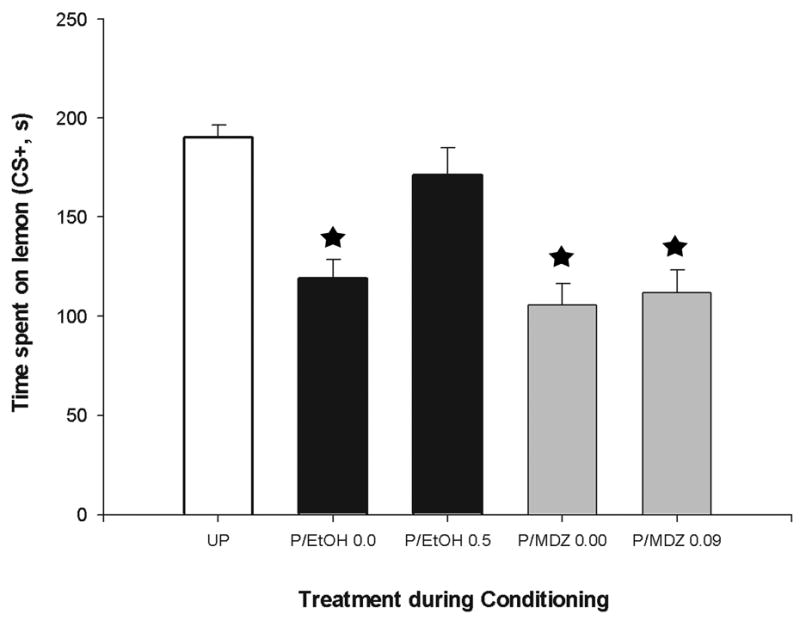
Time spent on lemon odor side (CS+) as a function of conditioning procedures (Paired or Unpaired presentations of lemon and intraoral infusion of citric acid, P and UP, respectively) and drug treatment received during devaluation [ethanol, EtOH (0.0 or 0.5 g/kg) or midazolam, MDZ (0.00 or 0.09 mg/kg)]. Unpaired groups have been collapsed across drug treatment due to the lack of significant differences between them. Vertical bars represent the standard error of the means (S.E.M.). Asterisks indicate significant differences from the Unpaired and Paired ethanol (0.5 g/kg) groups (p < 0.05).
There was no evidence of effects on locomotor activity at test from the drugs received by the animals 120 minutes before. Specifically, the ANOVA failed to detect significant main effects or significant interactions between the factors.
Experiment 2 b
The previous experiment showed differential effects of EtOH and MDZ on expression of a conditioned aversion in a devaluation paradigm. EtOH, but not MDZ, successfully devalued the expression of a citric-acid mediated odor avoidance response. This result was observed after drug dosage for EtOH and MDZ had been equated in terms of their suppression of USVs elicited by isolation and intraorally infused citric acid. This does not completely preclude the possibility that MDZ's failure to alter expression of aversive learning is related to dose-response factors. That is, it could be the case that a higher dose of MDZ is needed for the drug to successfully alter an odor-conditioned aversion of the magnitude observed in Experiment 2 a. This possibility was tested in the following experiment, in which a 2-fold higher dose of MDZ was employed. Pups were given a conditioned aversion to lemon odor by pairing it with citric acid infusions as in Experiment 2 a. One day later the devaluation phase took place: animals were briefly infused with the citric solution after having been administered with 0.18 mg/kg of MDZ. Pups were later tested for preference to lemon odor.
Methods
Experimental design and general procedures
Sixty one animals representing eight litters were randomly assigned to 4 groups defined as a function of the nature of the contingency between lemon odor exposure and intraoral infusion of citric acid (Groups paired and unpaired), and drug treatment at devaluation (MDZ, 0.00 or 0.18 mg/kg). Conditioning and testing procedures replicated those described in Experiment 2 a. That is, citric odor aversion training (PD 14) was followed by a brief devaluation procedure and subsequent two-way odor preference assessments (PD 15). Relative to Experiment 2 a, a higher dose of MDZ (0.18 mg/kg) was employed.
Data Analysis
As was the case in Experiment 2 a, odor preference scores (absolute and percent time spent over the lemon odor) in unpaired controls did not differ as a function of drug treatment during devaluation, F (1, 28) = 0.65, p = 0.43; F (1, 28) = 1.01, p = 0.32 Consequently, pups in these conditions were collapsed into a single control group (UP). Absolute and percent time spent over the lemon CS were examined by a one-way ANOVA, which took into account treatment during conditioning (UP, P/MDZ 0.18, P/MDZ 0.0) as the comparative factor between groups. A 2 × 2 ANOVA [learning condition (paired or unpaired) × treatment at devaluation (MDZ or vehicle)] served to analyze locomotion scores during the two-way odor preference test.
Results
Figure 3 presents absolute time spent in the section of the test cage closest to the lemon scent. Pups given the citric infusion while exposed to the lemon scent and later treated with vehicle during the devaluation stage displayed lower lemon preference scores than unpaired controls. Specifically, P/MDZ 0.0 pups spent a mean of 115 s on the lemon scented section of the test chamber, significantly less than the 190 s exhibited by UP controls. Apparently, administration with 0.18 mg/kg MDZ during the devaluation phase did not alter expression of the conditioned aversion. These observations were confirmed by ANOVA conducted on absolute and percent time over lemon odor, which indicated a significant main effect of treatment during conditioning, F (2, 58) = 28.37, F (2, 58) = 16,16; respectively, both p's < 0.0005. In both cases, LSD's post hoc tests showed that paired groups (P/MDZ 0.0 and P/MDZ 0.18) spent significantly less time over the lemon-scented section than UP counterparts. No significant difference between the paired groups was detected. Mean and standard error for percent time over lemon odor was as follows: Unpaired Controls = 70.72 ± 2.8, P/MDZ 0.0 = 49.72 ± 3.7, P/MDZ 0.18 = 48.78 ± 3.4.
Figure 3.

Time spent on lemon odor side (CS+) as a function of conditioning procedures (Paired or Unpaired presentations of lemon and intraoral infusion of citric acid, P and UP, respectively) and drug treatment received during devaluation (0.00 or 0.18 mg/kg of midazolam, MDZ). Unpaired groups have been collapsed across drug treatment due to the lack of significant differences between them. Vertical bars represent the standard error of the means (S.E.M.). Asterisks indicate significant differences from the Unpaired group (p < 0.05).
A significant effect of conditioning treatment was observed relative to the levels of locomotion registered at testing. Specifically, pups in paired groups displayed more locomotion than unpaired counterparts, F (1, 57) = 5.24; p< 0.05. Interestingly, locomotor patterns were not affected by the drug treatment received by the pups during the devaluation stage. No other main effects or interactions reached significance. Locomotor patterns at test are depicted in figure 4.
Figure 4.
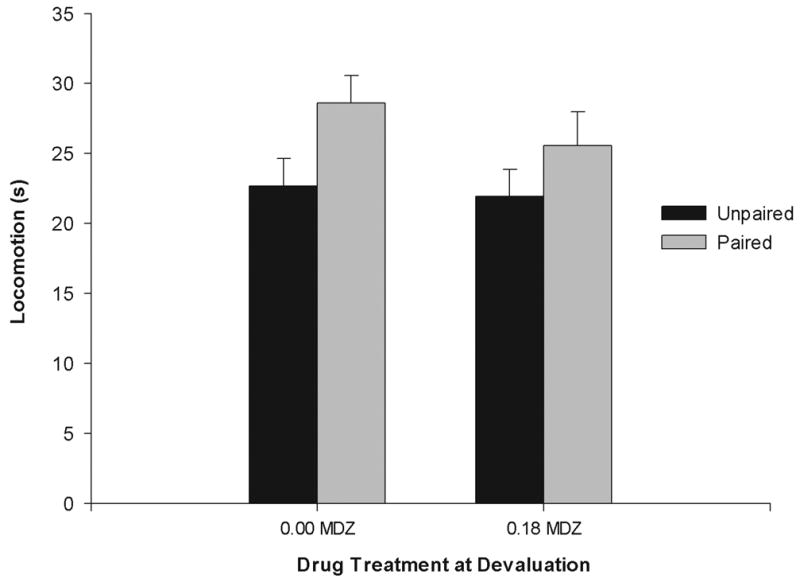
Locomotion scores during the 5-minute two-way odor preference test (seconds) as a function of conditioning procedures (Paired or Unpaired presentations of lemon and intraoral infusion of citric acid, P and UP, respectively) and drug treatment received during devaluation (0.00 or 0.18 mg/kg of midazolam, MDZ). Vertical bars represent the standard error of the means (S.E.M.).
In brief, Experiments 2 a and 2 b indicate that citric acid acts as an effective aversive unconditioned stimulus. In agreement with previous work (Molina et al., 1996) pups readily avoided an odor paired with citric acid intraoral infusion. Yet, brief exposure to the original aversive US under the influence of EtOH (0.5 g/kg) was sufficient to significantly inhibit expression of the avoidance response conditioned by citric acid. Apparently, ethanol devalued the representation of this aversive US, a result previously interpreted in terms of anxiolytic properties of the drug (Pautassi et al., 2006). This devaluating capability was not shared by the GABAA agonist midazolam at any of the doses under analysis (0.09 and 0.18 g/kg). This suggests that this receptor complex is not mediating EtOH's effects in this specific preparation. These results were not due to drug related changes in motor activity at test. In Experiment 2 b paired animals exhibited greater overall activity than unpaired controls. Yet, this result was independent of whether pups had experienced MDZ or vehicle at devaluation, indicating that residual effects of the drug on locomotion at test did not affect odor preference scores.
Discussion
The general aim of this work was to assess anxiolytic properties of EtOH in the early ontogeny of the rat as tested by unconditioned as well as conditioned behavior. EtOH's effects were compared with those of a GABA-mediated anxiolytic agent, midazolam. In particular, we wanted to test the claim that ethanol's effects in a devaluation paradigm, as seen by Pautassi et al. (2006), were GABA-mediated.
Experiment 1 indicated that pups exposed to certain stressors emitted more USVs than untreated controls. The combination of maternal isolation, intraoral citric acid and drug vehicle administration produced a significantly increased stress response relative to pups receiving isolation and citric acid in conjunction with handling alone. This suggests that vehicle administration is a critical stressor, which interacts with other treatments in evoking USVs. Enhanced USV production has been found by combining isolation-mediated stress with alternative stress sources, such as thermal challenges (Engel and Hard, 1987) as well as repeated episodes of maternal separation (Kraebel et al., 2002). The GABAA benzodiazepine agonist midazolam exerted a dramatic calming effect upon these distress calls, particularly at high doses (Experiment 1). To our knowledge, this is the first evidence showing the effectiveness of midazolam in reducing distress calls during infancy. Nobre and Brandao (2004) have shown such an effect during adulthood using shock as a US. Previous studies have also indicated reduced number of USVs in isolated rats administered with alternative benzodiazepines (diazepam and chlordiazepoxide; Gardner, 1985) or ethanol (0.5 – 1.0 g/kg; Engel and Hard, 1987). Experiment 1 agrees with the latter study in that administration of a low dose of ethanol (0.5 g/kg) exerts a calming effect upon USV emission. This EtOH-induced reduction was indistinguishable from that of 0.09 mg/kg MDZ. This experiment allowed us to equate EtOH's apparent anxiolytic effects with those of a specific midazolam dose. The results of Experiment 1 suggest that when employing this particular unconditioned model of anxiety, ethanol exerts anxiolytic effects early in life. Further work is needed to clarify the specific neural mechanisms underlying these results. However, many of the drugs that modulate USVs in a variety of experimental conditions activate the GABA system without affecting motor activity (for a review see: Winslow and Insel, 1991; infant studies: Gardner, 1985; Insel et al., 1986; Engel and Hard, 1987; adult studies: Beckett et al., 1986; Nobre and Brandao, 2004). Furthermore, Engel and Hard (1987) showed that ethanol's suppressing effects on USVs was inhibited by the GABAergic antagonist picrotoxin in infant rats. Overall, this converging body of evidence suggests that the GABA system is likely to be involved in the effects observed in Experiment 1.
After titrating EtOH and MDZ in terms of their capability to modulate an innate index of anxiety, these drugs were tested in an alternative model employing conditioned anxiety. Previously, Pautassi et al. (2006) reported that post-conditioning, low dose ethanol devalued the expression of an aversive memory by acting directly upon the representation of an aversive US (citric acid). In Experiment 2 we recreated this preparation to (a) further validate Pautassi et al.'s (2006) results and (b) to determine whether EtOH and midazolam would have similar devaluating effects. The latter result would suggest that activation of GABAA receptors underlies ethanol's devaluing capabilities. An alternative strategy could have been to employ a GABAA antagonist to block ethanol's devaluing effects. Midazolam was selected because this drug, like ethanol, is a positive allosteric modulator of the GABAA receptor that produces a robust anxiolytic action (Wilson et al., 2004). We considered the use of a GABAA receptor antagonist (i.e., picrotoxin) to assess whether this treatment would block ethanol's devaluing properties. However, an important concern was that such an antagonist might unnecessarily reduce alternative GABAA receptor-mediated behaviors (i.e., locomotor activity, Osborne et al., 1993) or even elicit effects (e.g., pain-like effects, Olivéras and Montagne-Clavel, 1994) that could interfere with ethanol's devaluing action. It would have been very difficult to know whether responding at testing was due to selective disruption of the anxiolytic properties of ethanol, or to non-selective blockage of other GABAA receptor mediated behaviors.
Experiment 2 indicated that few pairings between intraoral citric acid and a salient odor caused avoidance of this CS. In what constitutes a replication of Pautassi et al. (2006) results, this avoidance response was inhibited by a brief post-conditioning pairing of the original US (citric acid) and the early post-absorptive effects of 0.5 g/kg EtOH. Expression of the conditioned aversive response was not affected by midazolam (0.09 or 0.18 mg/kg; Experiments 2a and 2b, respectively). These effects were not due to changes in locomotor activity. Motor performance at test was not affected by drug administration conducted 120 minutes earlier. That is, EtOH's effects appear to be related to its motivational properties and not derived from motor activating or depressing effects of the drug. According to previous studies, blood ethanol concentrations (BECs) during devaluation are approximately equivalent to 50 mg%. At test blood ethanol levels drop to 20 mg% (Pautassi et al., 2005). According to prior studies these low concentrations of ethanol are not sufficient to disrupt sensory capabilities or to interfere with expression of conditioned avoidance learning (Molina et al., 1984; 1987).
In Experiment 2 b, locomotion was higher in animals that had experienced the odor CS paired with citric acid infusion. This can be interpreted as an alternative index of citric acid mediated aversive learning. An increase in locomotion is a rather common outcome when assessing either aversive conditioning or acute response to aversive stimuli (Arias and Chotro, 2006, Brining et al., 1991). However, this profile differed from Experiment 2a, where no differences in locomotion were found. This probably indicates that the apparent locomotor conditioned response effect is not very reliable under this particular set of experimental conditions.
Considered along with Pautassi et al.'s (2006) previous work, results of Experiment 2 clearly indicate that ethanol exerts powerful devaluation of an aversive memory induced by intraoral infusion of citric acid. However, the current results do not support a role for GABAA receptor systems in mediation of this effect. The benzodiazepine midazolam did not produce a similar devaluation effect on the response evoked by the CS. Nevertheless, we should be cautious in terms of completely dismissing a possible role of GABAA receptors due to the results described here. Although benzodiazepines and ethanol are both positive modulators of GABAA receptor function they may not bind the same sites on the GABAA receptor ionophore and if so would not necessarily be expected to produce the same actions on channel gating. Furthermore, recent evidence suggests that the behavioral pharmacology of specific GABAA receptors may depend on subunit composition (Boehm II et al., 2004; Rudolph and Mohler, 2004) Thus, if ethanol and midazolam produce their behavioral (anxiolytic) actions at GABAA receptors composed of different subunits it is possible that one cannot effectively substitute one for the other in the devaluation procedure. Given these possibilities, the conclusion regarding the lack of GABAA involvement in ethanol's devaluing capabilities should be considered as a preliminary working hypothesis. A conclusive answer to this question s will have to await determination of the precise binding site/s for ethanol on the GABAA receptor, as well as subunit specificity for ethanol's anxiolytic actions.
EtOH not only facilitates GABA transmission but also interacts with other transmitter systems, such as glutamate (Gonzalez and Jaworski, 1997; Manto et al., 2005). In fact, animals perceive the internal state induced by EtOH as similar to the one generated by dizocilpine, a NMDA antagonist. This generalization does not hold true for MDZ (Porcu and Grant, 2004). This suggests a commonality of effects between EtOH and NMDA antagonists. Interestingly, the latter compounds have been shown to exert anxiolytic effects in a variety of tests (Martinez et al., 2002; Molina-Hernández et al., 2006; Jessa et al., 1996). Therefore, it could be argued that early anxiolytic effects of EtOH act in the devaluation paradigm through NMDA mechanisms. Also, it has been observed that systemic ethanol enhances central levels of dopamine, a transmitter known to be involved in appetitive reinforcement (Ericson et al., 2003). This raises the possibility that the reduced aversive response toward the olfactory CS after devaluation with EtOH reflects competing appetitive reinforcing capabilities of the drug. Recently, it has been found that low dose ethanol (0.5 g/kg) supported appetitive conditioning in infant rats. Specifically, a flavor CS paired with an EtOH dose resulting in BECs strikingly similar to those likely to be present during devaluation (50 mg%) acted as a positive reinforcer when later associated with a tactile CS (Molina et al., 2006). Also, expression of EtOH's appetitive effects is facilitated when the drug is administered in conjunction with stressful stimuli (Matsuzawa et al., 1998). The devaluation paradigm employed in this work certainly involves ethanol administration in the context of unconditional stressful stimuli or CSs predicting these aversive events.
An alternative explanation for the results observed in Experiment 2 is that the lack of devaluation effects in the P/MDZ groups was due to lingering effects of MDZ on behavior at testing. Two points argue against this possibility. First, there was no drug effect upon locomotion activity at test. Second, the elimination half-life of MDZ has been observed to be 30 minutes (Kotegawa et al., 2002). Given this fact, at 120 minutes post-administration of a 0.09 or 0.18 mg/kg MDZ dose, only a trace amount of the drug should be present in the system. It also seems highly unlikely that low doses of the drug might produce alternative effects that compete with its putative devaluing properties, at least with regard to MDZ amnesic effects. Specifically, doses much larger than those employed in the present work are needed for the drug to exert detrimental effects upon memory formation (e.g., 3 mg/ kg, Pain et al., 2002; 5 mg/kg, Semba et al., 2005).
In summary, the results of this study indicate similar calming effects of EtOH and midazolam when assessed through an unconditional measure of anxiety (social isolation and citric-acid induced USVs). Subsequently, it was confirmed that EtOH can devalue the representation of an aversive US and, hence, the magnitude of the conditioned aversion to this stimulus. Midazolam did not share this devaluation effect, even when doses of these drugs were adjusted to equate their suppression of USVs induced by citric acid. This indicates that the devaluation effects of EtOH are probably not mediated by the GABA system. Appetitive motivational properties of EtOH or non-GABA-mediated anti-anxiety effects (i.e, NMDA-related) could underlie this devaluation effect of ethanol.
Acknowledgments
This work was supported by Supported by grants from the NIAAA (AA11960, AA013098) and the NIMH (MH035219) to NES and the Agencia Nacional de Promocion Cientifica y Tecnologica (PICT 05-14024) to JCM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdi H. The Bonferonni and Šidák Corrections for Multiple Comparisons. In: Salkind Neil., editor. Encyclopedia of Measurement and Statistics. Thousand Oaks, CA: Sage; 2007. [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nature Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Ethanol-Induced Preferences or Aversions as a Function of Age in Preweanling Rats. Behav Neurosci. 2006;120:710–718. doi: 10.1037/0735-7044.120.3.710. [DOI] [PubMed] [Google Scholar]
- Bechthold A, Cunningham CL. Ethanol-Induced Conditioned Place Preference Is Expressed Through a Ventral Tegmental Area Dependent Mechanism. Behav Neurosci. 2005;119:2213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- Beckett SRG, Aspley S, Graham M, Marsden CA. Pharmacological manipulation of ultrasound induced defensive behavior in the rat. Psychopharmacology. 1996;127:384–386. doi: 10.1007/s002130050102. [DOI] [PubMed] [Google Scholar]
- Bianchi MS, Lux-Lantosa VA, Bettler B, Libertun C. Expression of gamma-aminobutyric acid B receptor subunits in hypothalamus of male and female developing rats. Dev Brain Res. 2005;160:124–129. doi: 10.1016/j.devbrainres.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Ponomarev I, Jennings AW, Whiting PJ, Rosahl TW, Garrett EM, Blednov YA, Harris RA. γ-Aminobutyric Acid A receptor subunit mutant mice: new perspectives on alcohol actions. Biochem Pharmacol. 2004;68:1581–1602. doi: 10.1016/j.bcp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125:49–56. doi: 10.1016/s0166-4328(01)00277-7. [DOI] [PubMed] [Google Scholar]
- Brining SK, Belecky TL, Smith DV. Taste reactivity in the hamster. Physiol Behav. 1991;49:1265–1272. doi: 10.1016/0031-9384(91)90361-q. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-Induced Conditioned Taste Aversion in 15 Inbred Mouse Strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- Bustos SH, Maldonado H, Molina VA. Midazolam disrupts fear memory reconsolidation. Neuroscience. 2006;139:831–42. doi: 10.1016/j.neuroscience.2005.12.064. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Hill K. Animal's Models of Alcohol's Motivational Effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- Dirks A, Fish EW, Kikusui T, Van der Gugten J, Groenink L, Olivier B, Miczek KA. Effects of corticotropin-releasing hormone on distress vocalizations and locomotion in maternally separated mouse pups. Pharm Biochem Behav. 2002;72:993–999. doi: 10.1016/s0091-3057(02)00809-2. [DOI] [PubMed] [Google Scholar]
- Engel J, Hard E. Effects of diazepam, ethanol and Ro 15-1788 on ultrasonic vocalization, locomotor activity and body righting in the neonatal rat. Alcohol Alcohol Suppl. 1987;1:709–712. [PubMed] [Google Scholar]
- Fernandez-Vidal JM, Molina JC. Socially mediated alcohol preferences in adolescent rats following interactions with an intoxicated peer. Pharmacol Biochem Behav. 2004;79:229–241. doi: 10.1016/j.pbb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Gardner CR. Inhibition of ultrasonic distress vocalizations in rat pups by chlordiazepoxide and diazepam. Drug Dev Res. 1985;5:185–193. [Google Scholar]
- Gonzalez RA, Jaworski JN. Alcohol Res. Health. 1997;21:120–127. [PMC free article] [PubMed] [Google Scholar]
- Hitzemann R. Animal Models of Psychiatric Disorders and Their Relevance to Alcoholism. Alcohol Res Health. 2000;24:149–158. [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hill JL, Mayor RB. Rat pup ultrasonic isolation calls: Possible mediation by the benzodiazepine receptor complex. Pharmacol Biochem Behav. 1986;24:1263–1267. doi: 10.1016/0091-3057(86)90182-6. [DOI] [PubMed] [Google Scholar]
- Jessa M, Nazar M, Bidzinski A, Plaznik A. The effects of repeated administrations of diazepam, MK-801 and CGP 37849 on rat behavior in two models of anxiety. Eur Neuropsychopharmacol. 1996;6:55–61. doi: 10.1016/0924-977x(95)00068-z. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kraebel KS, Brasser SM, Campbell JO, Spear LP, Spear NE. Developmental differences in temporal patterns and potentiation of isolation-induced ultrasonic vocalizations: influence of temperature variables. Dev Psychobiol. 2002;40:147–59. doi: 10.1002/dev.10022. [DOI] [PubMed] [Google Scholar]
- Kotegawa T, Laurijssens BE, Von Moltke LL, Cotreau MM, Perloff MD, Venkatakrishnan K, Warrington JS, Granda BS, Harmatz JS, Greenblatt DJ. In Vitro, Pharmacokinetic, and Pharmacodynamic Interactions of Ketoconazole and Midazolam in the Rat. J Pharmacol Exp Ther. 2002;302:1228–1237. doi: 10.1124/jpet.102.035972. [DOI] [PubMed] [Google Scholar]
- Kubova H, Mares P. The effect of ontogenetic development on the anticonvulsant activity of midazolam. Life Sci. 1992;50:1665–1672. doi: 10.1016/0024-3205(92)90421-k. [DOI] [PubMed] [Google Scholar]
- Manto M, Laute MA, Pandolfo M. Depression of extracellular GABA and increase of NDMA-induced nitric oxide following acute intra-nucelar administration of alcohol in the cerebellar nuclei of the rat. Cerebellum. 2005;4:230–238. doi: 10.1080/14734220500243835. [DOI] [PubMed] [Google Scholar]
- Martínez G, Ropero C, Funes A, Flores E, Blotta C, Landa AI, Gargiulo PA. Effects of selective NMDA and non-NMDA blockade in the nucleus accumbens on the plus-maze test. Physiol Behav. 2002;76:219–224. doi: 10.1016/s0031-9384(02)00704-7. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M. Conditioned fear stress induces ethanol-associated place preference in rats. Eur J Pharmacol. 1998;12:127–30. doi: 10.1016/s0014-2999(97)01456-8. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Flaherty C. Temporal dynamics of corticosterone elevation in successive negative contrastnext term. Physiol Behav. 1998;64:287–292. doi: 10.1016/s0031-9384(98)00072-9. [DOI] [PubMed] [Google Scholar]
- Molina JC, Serwatka J, Enters K, Spear LP, Spear NE. Acute alcohol intoxication disrupts brightness but not olfactory conditioning in preweanling rats. Behav Neurosci. 1987;101:846–853. doi: 10.1037//0735-7044.101.6.846. [DOI] [PubMed] [Google Scholar]
- Molina JC, Serwatka J, Spear NE. Changes in alcohol intake resulting from prior experience with alcohol odor in young rats. Pharmacol Biochem Behav. 1984;21:387–391. doi: 10.1016/s0091-3057(84)80100-8. [DOI] [PubMed] [Google Scholar]
- Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol's motivational effects: Ethanol reinforcement during the third postnatal week. Alcohol Clin Exp Res. 2006;30:1506–1519. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- Molina JC, Bannoura MD, Chotro MG, Mckinzie DL, Moore A, Spear NE. Alcohol mediated tactile conditioned aversions in infants rats: Devaluation of conditioning through alcohol-sucrose associations. Neurobiol Learn Mem. 1996;66:121–132. doi: 10.1006/nlme.1996.0053. [DOI] [PubMed] [Google Scholar]
- Molina-Hernández M, Tellez-Alcántara NP, Pérez-García J, Olivera-Lopez JI, Jaramillo T. Antidepressant-like and anxiolytic-like actions of the mGlu5 receptor antagonist MTEP, microinjected into lateral septal nuclei of male Wistar rats. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1129–1135. doi: 10.1016/j.pnpbp.2006.04.022. [DOI] [PubMed] [Google Scholar]
- National Institute of Health. National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. Institute of Laboratory Animal Resources, Commission on Life Sciences. [Google Scholar]
- Nobre MJ, Brandao ML. Analysis of freezing behavior and ultrasonic vocalization in response to foot-shocks, ultrasound signals and GABAergic inhibition in the inferior colliculus: Effects of muscimol and midazolam. European Neuropsychopharmacology. 2004;14:45–52. doi: 10.1016/s0924-977x(03)00073-7. [DOI] [PubMed] [Google Scholar]
- Olivéras JL, Montagne-Clavel J. The GABAA receptor antagonist picrotoxin induces a ‘pain-like’ behavior when administered into the thalamic reticular nucleus of the behaving rat: a possible model for ‘central’ pain? Neurosci Lett. 1994;179:21–24. doi: 10.1016/0304-3940(94)90925-3. [DOI] [PubMed] [Google Scholar]
- Olivier B, Molewijk E, Van Oorschot R, Van der Poel G, Zethol T, Van der Hieden JY, Mos J. New animal models of anxiety. Eur Neuropsychopharmacol. 1994;4:93–112. doi: 10.1016/0924-977x(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Osborne PG, Mataga N, Onoe H, Watanabe Y. Behavioral activation by stimulation of a GABAergic mechanism in the preoptic area of rat. Neurosci Lett. 1993;158:201–204. doi: 10.1016/0304-3940(93)90264-l. [DOI] [PubMed] [Google Scholar]
- Pain L, Angst MJ, LeGourrier L, Oberling P. Effect of a nonsedative dose of propofol on memory for aversively loaded information in rats. Anesthesiology. 2002;97:447–453. doi: 10.1097/00000542-200208000-00023. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Godoy JC, Spear NE, Molina JC. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin Exp Res. 2002;26:644–654. [PubMed] [Google Scholar]
- Pautassi RM, Melloni C, Ponce LF, Molina JC. Acute ethanol counteracts the acquisition of aversive olfactory learning in infant rats. Alcohol. 2005;36:99–105. doi: 10.1016/j.alcohol.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Sanders S, Miller S, Spear NE, Molina JC. Early ethanol's anxiolytic effects assessed through an unconditional stimulus revaluation procedure. Alcohol Clin Exp Res. 2006;30:448–459. doi: 10.1111/j.1530-0277.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: Age-Related Changes in the Rewarding and Aversive Effects of Alcohol. Alcohol Clin Exp Res. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Porcu P, Grant KA. Discriminative stimulus effects of ethanol in rats using a three-choice ethanol-midazolam-water discrimination. Behav Pharm. 2004;15:555–567. doi: 10.1097/00008877-200412000-00004. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Semba K, Adachi N, Arai T. Facilitation of serotonergic activity and amnesia in rats caused by intravenous anesthetics. Anesthesiology. 2005;102:616–23. doi: 10.1097/00000542-200503000-00021. [DOI] [PubMed] [Google Scholar]
- Scalera G. Acid taste thresholds assessed by conditioned taste aversion and two-bottle preference in rats. Physiol Behav. 2004;82:411–423. doi: 10.1016/j.physbeh.2004.04.052. [DOI] [PubMed] [Google Scholar]
- Spear LP, Specht SM, Kirstein CL, Kuhn CM. Anterior and posterior, but not cheek, intraoral cannulation procedures elevate serum corticosterone levels in neonatal rat pups. Dev Psychobiol. 1989;22:401–411. doi: 10.1002/dev.420220407. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Burghardt PR, Ford KA, Wilkinson MB, Primeaux SD. Anxiolytic effects of diazepam and ethanol in two behavioral models: comparison of males and females. Pharmacol Biochem Behav. 2004;78:445–458. doi: 10.1016/j.pbb.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Infant rat separation is a sensitive test for novel anxiolytics. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:745–757. doi: 10.1016/0278-5846(91)90003-j. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Yamamoto T. Effects of midazolam on the expression of conditioned taste aversion in rats. Brain Res. 2005;1043:115–123. doi: 10.1016/j.brainres.2005.02.070. [DOI] [PubMed] [Google Scholar]