Abstract
Classically, the extracellular matrix (ECM) was viewed as a supporting structure for stabilizing the location of cells in tissues and for preserving the architecture of tissues. This conception has changed dramatically over the past few decades with discoveries that ECM has profound influences on the structure, viability, and functions of cells. Much of the data supporting this new paradigm has been obtained from studies of normal and pathological structural cells such as fibroblasts, smooth muscle cells, and malignant cells, as, for example, breast cancer epithelial cells. However, there has also been recognition that effects of ECM on cells extend to inflammatory cells. In this context, attention has been drawn to fragments of ECM components. In this review, we present information supporting the concept that proteolytic fragments of ECM affect multiple functions and properties of inflammatory and immune cells. Our focus is particularly upon neutrophils, monocytes, and macrophages and fragments derived from collagens, elastin, and laminins. Hyaluronan fragments, although they are not products of proteolysis, are also discussed, as they are a notable example of ECM fragments that exhibit important effects on inflammatory cells. Further, we summarize some exciting recent developments in this field as a result of mouse models in which defined ECM fragments and their receptors are clearly implicated in inflammation in vivo. Thus, this review underscores the idea that proteolysis of ECM may well have implications that go beyond modifying the structural environment of cells and tissues.
Keywords: matrikine, collagen peptides, elastin peptides, laminin peptides, hyaluronan fragments, elastin receptor, SIKVAV
1. Introduction
Extracellular matrix (ECM) is a complex mixture of proteins, proteoglycans, and glycosaminoglycans that supports cells and tissue architecture. In recent decades it has become apparent that ECM also provides signals affecting cell adhesion, shape, migration, proliferation/survival, and differentiation. Thus, ECM components have domains that interact with specific cell surface receptors. Classic examples of ECM interactions with cells involve the cell surface receptor family of integrins. However, there are also non-integrin based interactions between ECM and cell surface receptors. The ligand domain in an ECM component may be cryptic, that is, exposed only after the ECM is modified. These bioactive ECM domains, designated “matricryptins” (Davis, 2000; Schenk, 2003), have functions distinct from the parent molecule. Proteolytically released ECM fragments with bioactivity are called “matrikines” (Duca, 2004; Maquart, 2004).
Matrikines in wound healing and tumor progression have been the topic of several recent reviews (Hornebeck, 2003; Duca, 2004; Maquart, 2004; Tran, 2004; Labat-Robert, 2005; Maquart, 2005; Labat-Robert, 2007). This brief commentary reviews recent developments in the expanding topic of ECM fragments as mediators of inflammation. Although degradation of ECM may affect inflammation via release of cytokines associated with the ECM (Alon, 1994; Hershkoviz, 1994; Hershkoviz, 1995; Vaday, 2001), this aspect of ECM and inflammation is beyond the scope of this review. This review will focus on the pro- and anti-inflammatory effects of ECM fragments, with emphasis on fragments generated from laminins, collagens, elastin, and hyaluronan. First, in vitro evidence for inflammatory effects of ECM fragments is summarized; then results from recent in vivo studies are presented.
2. In Vitro Analysis of the Effects of ECM Fragments on Inflammatory Cells: General Comments
Many in vitro studies involving either proteolytic ECM fragments or synthetic peptides corresponding to ECM sequences indicate that ECM fragments can affect inflammatory cells. The applicability of such studies to inflammatory processes in vivo is uncertain, however, because these types of studies typically have been simple, involving a single ECM fragment in solution and a single cell type. Despite their limitations, studies of this type can be useful in a number of ways: they demonstrate the presence and signaling of receptors for ECM-derived ligands; they provide information about ECM receptor numbers and affinity for specific ECM-derived ligands; they yield an opportunity to investigate signaling pathways triggered by ligand binding; and they enable analysis of the effects of ligand binding on gene expression.
However, degradation of ECM in vivo is a complex process, involving release of fragments from a variety of ECM molecules concurrently. Moreover, many proteases may be involved in the degradation process, even for an individual ECM component. Therefore, the pro-inflammatory or anti-inflammatory role of fragments from a specific ECM component may be difficult to discern. Examination of the inflammatory response while blocking the receptor(s) for a specific ECM fragment helps to define the potential role of these fragments in tissue injury, but it is possible that a given receptor has other ligands, making the interpretation of these experiments difficult. Alternative ways to assess the impact of ECM ligands include using fragment-specific blocking antibodies or by generating mice in which the ligand binding site within the ECM molecule of interest has been mutated.
2.1 ECM-derived fragments exhibit chemotactic activity for inflammatory cells
The list of ECM-derived peptides reported to have chemotactic activity for inflammatory cells is lengthy and the data on which such a compilation is based have been collected over many years. A partial list includes collagen types I and IV, elastin, fibronectin, laminins, entactin/nidogen, thrombospondin, and hyaluronan. Noteworthy is that the chemotaxis dose response curves to ECM fragments can be comparable to those obtained with classic chemoattractants such as formyl met-leu-phe (fMLP) and the complement anaphylatoxin C5a (Senior, 1989). Accordingly, it is plausible that some fragments of ECM might actually recruit inflammatory cells in vivo.
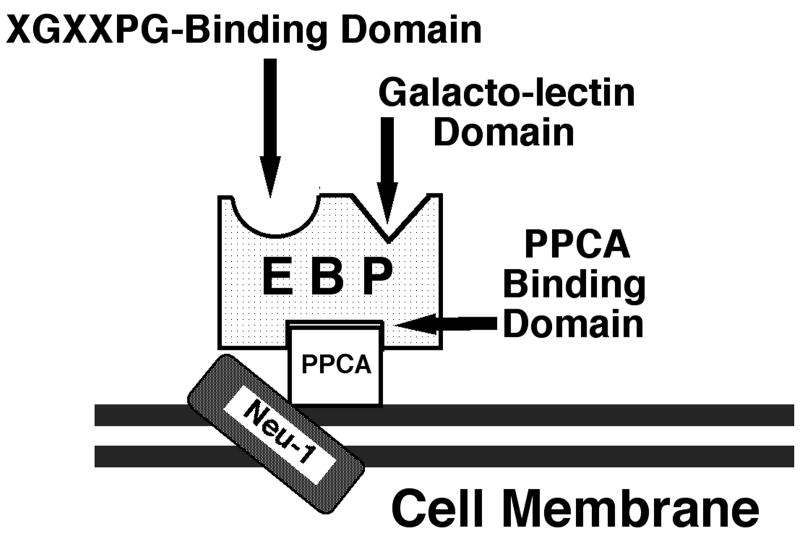
Various cell surface receptors are responsible for inflammatory cell chemotaxis to ECM fragments. Neutrophils exhibit chemotaxis to fragments of type IV collagen, laminins, and elastin using a 67-kDa protein (Senior, 1989). This receptor recognizes X-Gly-X-X-Pro-Gly (XGXXPG) motifs found in several ECM components. The identity of this receptor protein, commonly called the elastin binding protein (EBP), has been established as an enzymatically inactive, alternatively spliced variant of β-galactosidase. EBP forms a complex with protective protein/cathepsin A (PPCA) and lysosomal sialidase (neuraminidase-1, Neu-1) (Fig. 1). Recent studies have shown that the Neu-1component of the EBP complex is responsible for triggering cellular activation (Duca, 2007). EBP is present on many cell types, including various types of leukocytes, mesenchymal cells, vascular smooth muscle cells, and skin fibroblasts (Hinek, 1996; Malvagia, 2004; Larbi, 2005; Tatano, 2006; Duca, 2007).
Fig. 1. The elastin receptor complex.
A 67-kDa enzymatically inactive, alternatively spliced variant of human β-galactosidase is identical to the previously described elastin binding protein (EBP). This protein binds the X-Gly-X-X-Pro-Gly (XGXXPG) motif in ECM proteins, such as elastin, laminins, collagen type IV, and fibrillin-1. EBP complexes with the 55-kDa protective protein-cathepsin A (PPCA) and the 61-kDa neuraminidase (Neu-1) on the cell surface. This receptor complex, after interaction with XGXXPG motifs, via Neu-1, transduces intracellular signals that trigger numerous cellular responses (provided by Aleksander Hinek, see (Hinek, 1988; Hinek, 1996; Privitera, 1998)).
From a given ECM component more than one receptor may be involved in stimulating chemotactic responses. Neutrophils, for example, have other type IV collagen binding molecules besides the EBP complex, including L-selectin (Iwabuchi, 1996). Moreover, while 7S domains of type IV collagen chain have neutrophil chemotactic activity via the EBP complex (Senior, 1989), a peptide from the α3 chain of type IV collagen is reported to suppress neutrophil activation (Monboisse, 1994). Thus, fragmentation of an individual ECM component may liberate fragments with opposing activities.
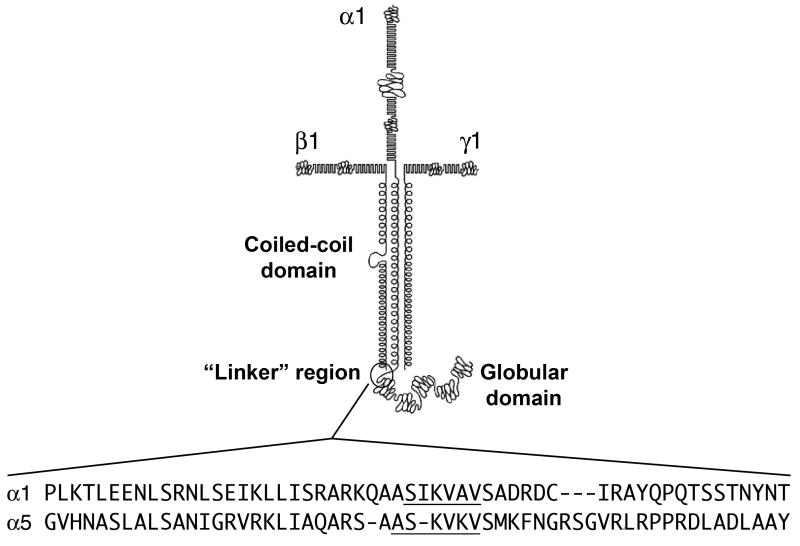
Other basement membrane components besides type IV collagen also elicit neutrophil chemotaxis. Entactin/nidogen has chemotactic activity that is integrin-mediated (Senior, 1992). A 140-kDa fragment encompassing the C-terminal end of the coiled-coil domain and the globular domain of laminin-111 generated by neutrophil elastase cleavage stimulates neutrophil migration in vitro (Steadman, 1993). Additional studies determined that a peptide containing the Ser-Ile-Lys-Val-Ala-Val (SIKVAV) sequence in the laminin α1 chain in the “linker” region between the coiled-coil and globular domains, as well as corresponding sequence Ala-Ser-Lys-Val-Lys-Val (ASKVKV) in the laminin α5 chain (Fig. 2), are chemotactic for neutrophils and macrophages in vitro (Adair-Kirk, 2003). The receptors for these ligands on inflammatory cells have not been determined, but SIKVAV has been shown to interact with integrins on a salivary gland carcinoma cell line (Freitas, 2007).
Fig. 2. The “linker” regions of the mouse laminin α chains.
The amino acid sequences of the “linker” region between the coiled-coil and the globular domains of two of the five the mouse laminin α chains are compared. Dashes are incorporated to maintain alignment. The SIKVAV-like sequences (underlined) in these laminin chains activate inflammatory cells (Corcoran, 1995; Khan, 1997; Khan, 2000; Adair-Kirk, 2003). Modified from (Adair-Kirk, 2003) with permission; Copyright 2003 The American Association of Immunologists, Inc.
Recent studies have extended our understanding of collagenous peptides as chemoattractants. Weathington et. al. discovered that acetylated Pro-Gly-Pro (acPGP), known to be a potent neutrophil chemoattractant derived from type I collagen in vivo, shares structural homology to a domain on ELR+ CXC chemokines and utilizes CXC chemokine receptors CXCR1 and CXCR2 (Weathington, 2006). Thus, some ECM-derived fragments promote chemotaxis by binding to cytokine receptors.
2.2 ECM-derived fragments enhance phagocytic functions
Bacteria can bind to ECM proteins through so-called MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) and utilize this binding as an early step in colonization and subsequent infection (Patti, 1994; Kreikemeyer, 2004). However, adhesion of phagocytes to ECM components may facilitate removal of bacteria by localizing them to the site of infection. In addition, adhesion of phagocytes to various ECM components (fibronectin, fibrinogen, vitronectin, collagen, entactin, and laminin-111) enhance phagocytic functions, such as ingestion and oxidative burst (Bohnsack, 1985; Brown, 1986; Senior, 1986; Monboisse, 1987; Brown, 1988; Parker, 1988; Yonemasu, 1988; Pike, 1989; Hermann, 1990; Laurent, 1991; Senior, 1992; Yang, 1994; Simms, 1997; Ottonello, 1998). Furthermore, the presence of cytokines within the ECM enhances not only the adhesion (Alon, 1994; Hershkoviz, 1994; Hershkoviz, 1995; Vaday, 2001), but also the bactericidal activities of phagocytes (Simms, 1997).
Synthetic peptides corresponding to sequences in laminins, fibronectin, vitronectin, and thrombospondin, which contain a heparin-binding motif, can mediate direct bacterial killing in a similar fashion as the cathelicidin class of antimicrobial peptides (Andersson, 2004; Malmsten, 2006). Together, these data indicate a potential role for ECM proteins in enhancement of the host defense against infections caused by pathogenic microorganisms.
2.3 ECM fragments induce immune responses
Fragments of ECM components exert effects on immune cells and elicit immune responses that may have a role in the pathogenesis of diseases involving ECM remodeling. In this context, recent observations with elastin-derived peptides are notable. With lymphocytes, elastin peptides foster Th-1 type differentiation and enhance the expression of Th-1 type cytokines in response to phytohemagglutinin (Debret, 2005). These effects occur coincident with increased expression of EBP and appear to require occupancy of the EBP as they can be blocked by anti-EBP antibody or by lactose that sheds EBP from the cell surface. In studies of monocytes, elastin peptides have the effect of blunting the response of the cells to lipopolysaccharide (LPS) stimulation (Baranek, 2007). The investigators speculate that this negative regulation is due to effects of elastin peptides on LPS receptors (CD14 and TLR-4), by so-called trans regulation of adjacent receptors.
Elastin-derived peptides may impact adaptive immunity. Higher levels of circulating anti-elastin antibody have been reported in individuals with smoking-associated pulmonary emphysema relative to controls (Lee, 2007). In these same studies, stimulation of T cells with elastin peptides resulted in interferon γ release that correlated with the severity of emphysema. From these observations, it is proposed that smoking elicits an inflammatory response that leads to release of proteases that degrade lung elastin. In individuals susceptible to emphysema, elastin peptides elicit immune responses leading to expression of cytokines that propel the inflammatory response. The net result is further release of proteases and progressive destruction of the lung ECM.
2.4 ECM-derived fragments induce changes in gene expression of inflammatory cells
Specific interactions between inflammatory cells and ECM components have been known for a long time, yet relatively little has been done to assess the effects of ECM fragments on inflammatory cell gene expression. Most studies in this area concern the effects of ECM fragments on monocyte/macrophage production of proteases or cytokines. Thus, for example, low molecular weight fragments of hyaluronan increase the expression of matrix metalloproteinase (MMP)-12, plasminogen activator inhibitor (PAI)-1 (Horton, 1999; Horton, 2000), and stimulate the production of several cytokines, including MIP-1α, MIP-1β, MCP-1, KC, IL-8, and IL-12 by macrophages (McKee, 1996; Hodge-Dufour, 1997; Horton, 1998a; Horton, 1998b). Several fibronectin-derived fragments increase monocyte/macrophage secretion of proteases (e.g., MMP-9/gelatinase B, MMP-12/macrophage elastase) and pro-inflammatory cytokines (e.g., IL1, IL6, and TNFα) (Beezhold, 1992; Xie, 1993; Lopez-Moratalla, 1995; Marom, 2007), whereas intact fibronectin does not have these effects, indicating that sites within these fragments are cryptic.
Peptides containing the SIKVAV sequence of laminin-111 induce the expression of MMP-9 and urokinase-type plaminogen activator (uPA) by monocytes/macrophages (Corcoran, 1995; Khan, 1997; Khan, 2000; Adair-Kirk, 2003), and a similar peptide (ASKVKV) derived from laminin-511 induces MMP-9 and MMP-14/MT1-MMP production by monocytes and macrophages, and MMP-9 release from neutrophilic granules (Adair-Kirk, 2003). Additional studies using microarray analysis indicated that this laminin α5-derived peptide up-regulates many genes, including the pro-inflammatory cytokine TNFα and one of its receptors, TNFR-II (Table 1) (Adair-Kirk, 2005). Of note, the chemotactic elastin-derived peptide Val-Gly-Val-Ala-Pro-Gly (VGVAPG), which is repeated several times in human elastin, failed to increase TNFα production by macrophages. These studies indicate that fragments derived from various ECM components can produce some similar responses in inflammatory cells, such as chemotaxis, but may have very different effects on gene expression.
Table 1. Microarray Detection of Signaling Ligand and Receptor mRNAs Increased at Least 3-fold in RAW264.7 Macrophages in Response to the Laminin α5 Peptide AQARSAASKVKVSMKF.
| Genbank # | Description | Fold Change |
|---|---|---|
| X70058 | Chemokine (C-C motif) ligand 7 (CCL7; MCP-3) | 26.7 |
| D86238 | Neuropeptide Y receptor Y2 (Npy2r) | 22.8 |
| X53798 | Chemokine (C-X-C motif) ligand 2 (CXCL2; MIP-2; Groβ) | 15.7 |
| M33266 | Chemokine (C-X-C motif) ligand 10 (CXCL10; IP-10) | 7.9 |
| X87128 | p75 TNF receptor (TNFR-II; Tnfrsf1b; CD120b) | 5.7 |
| M13926 | Colony stimulating factor 3 (granulocyte; Csf3) | 5.4 |
| X62502 | Macrophage inflammatory protein 1b (CCL4; MIP-1β) | 4.2 |
| U06924 | Signal transducer and activator of transcription 1 (STAT-1) | 3.9 |
| M19681 | Platelet-derived growth factor-inducible protein (CCL2; MCP-1) | 3.7 |
| D84196 | Tumor necrosis factor alpha (TNFα) | 3.5 |
| U29678 | Chemokine (C-C motif) receptor 1 (CCR1) | 3.4 |
| AI838195 | Opioid growth factor receptor (Ogfr) | 3.3 |
| J04491 | Chemokine (C-C motif) ligand 3 (CCL3; MIP-1α) | 3.2 |
Taken from (Adair-Kirk, 2005) with permission; Copyright 2005 The American Association of Immunologists, Inc.
Finding that ECM-derived fragments lead to changes in inflammatory cell gene expression has stimulated analysis of intracellular pathways involved in these effects. With peripheral blood lymphocytes, elastin peptides elicit AP-1 DNA binding (Debret, 2005). Using melanoma cells as the target cell and induction of IL-1β as the readout, Debret et. al. found that VGVAPG induced NF-κB translocation and DNA binding (Debret, 2006).
3. In Vivo Effects of ECM Fragments on Inflammatory Cells: General Comments
As described above, there is considerable evidence that fragments or peptides derived from ECM components modulate many activities of inflammatory cells in vitro. To determine whether these findings apply in vivo is difficult. Several pieces of data are needed: 1) induction of inflammatory effects when ECM-derived fragments or synthetic ECM domains are introduced into tissues; 2) detection of similar ECM fragments in biological samples, such as tissue extracts, serum, synovial fluid, or bronchoalveolar lavage fluid, coincident with tissue inflammation; and 3) demonstration that blocking the ligand binding site in the ECM molecule, typically by use of fragment-specific blocking antibodies, significantly alters the inflammation and/or tissue injury induced by the ECM ligand.
In trying to assemble these types of data, one needs to recognize that the inflammatory response that develops in vivo following instillation of an ECM fragment may not be a direct result of the ECM fragment, and that the degradation of the ECM in vivo is complex, involving multiple proteases, so that ECM fragments released in vivo in response to injury are likely to be different that those generated in vitro using a single protease. Therefore, additional studies need to be performed to show that the ECM fragment generated in vivo induces pro-inflammatory activities and that the activities are due to the previously identified site.
3.1 Laminin-derived peptides promote inflammatory cell protease production and migration
A SIKVAV-containing laminin α1 fragment recovered from human abdominal aortic aneurysm tissue was found to stimulate macrophage expression of urokinase-type plaminogen activator (uPA) and MMP-9 (Faisal Khan, 2002). In addition, instillation of a SIKVAV-like laminin α5-derived peptide into the lungs of mice resulted in robust recruitment of neutrophils within 1 day and macrophages by 3 days post-instillation (Adair-Kirk, 2003), and a concurrent increase in the MMP-9 was detected in the bronchoalveolar lavage fluid. This in vivo chemotactic response to the laminin α5 peptide is believed to be predominantly due to its ability to induce various cytokines by macrophages (Table 1) rather than the modest chemotactic activity of the laminin α5 peptide itself (Adair-Kirk, 2005). Together, these data show that laminin peptides may induce inflammatory cell recruitment and protease production in vivo.
3.2 Elastin fragments promote monocyte/macrophage recruitment in vivo
Chronic inflammation and elastin degradation are key features of abdominal aortic aneurysms, chronic obstructive pulmonary disease, and Marfan syndrome. Several studies have shed light into mechanisms by which macrophages are recruited into tissues during the development of these diseases. For example, extracts of abdominal aortic aneurysm specimens from patients undergoing surgical repair were shown to promote monocyte migration in vitro, which was blocked by incubation of the extracts with the BA4 monoclonal antibody, an antibody raised to bovine tropoelastin that recognizes the VGVAPG epitope (Hance, 2002). Furthermore, exposure of monocytes to lactose, to specifically induce dissociation of the EBP from the cell surface (Hinek, 1988), eliminated monocyte migration to aneurysm extracts. Accordingly, these data suggest that the chemotactic activity in the aneurysm extracts was due to in vivo derived elastin fragments. Similarly, aortic extracts from mgR/mgR mice, a model of Marfan syndrome, were shown to have macrophage chemotactic activity that could be blocked with BA4 or by pre-incubation of the macrophages with lactose prior to the chemotaxis assay (Guo, 2006). Together, these data implicate that elastin fragments are generated in vivo in aortic disease and that they possess chemotactic activity. However, they do not show that these elastin fragments play a role in the disease process.
Recently, Houghton et. al. observed a direct role for elastin fragments in triggering an inflammatory response in the lungs and subsequent tissue damage. Instillation of elastin fragments from bovine ligament (generated in vitro by digestion of the ligament with pancreatic elastase) into the lungs of mice induced monocyte, but not neutrophil, recruitment (Houghton, 2006). In addition, bronchoalveolar lavage fluid and lung homogenates of mice exposed to cigarette smoke induced monocyte migration in vitro, and this activity was blocked by BA4. To prove that the generation of elastin fragments in vivo facilitated the inflammatory response and lung tissue damage, BA4 was given concurrently with daily cigarette smoke exposure or following instillation of pancreatic elastase, another mouse model of emphysema. BA4 prevented the macrophage accumulation in response to cigarette smoke exposure or following instillation of pancreatic elastase. Furthermore, BA4 prevented additional, macrophage-mediated lung damage following pancreatic elastase instillation. These data indicate that elastin fragments generated in these forms of lung injury promote an inflammatory response and tissue damage. However, because BA4 reacts with XGXXPG-containing peptides that interact with the EBP, XGXXPG-containing fragments from ECM proteins other than elastin might also be involved in the pathologic processes observed.
3.3 A collagen-derived peptide promotes neutrophil migration in vivo
Another clear example of an ECM-derived peptide that is generated in vivo, and contributes to an in vivo inflammatory response, is the Pro-Gly-Pro (PGP) peptide from type 1 collagen. About 20 years ago, collagen fragments or collagen-derived peptides were shown to promote neutrophil chemotactic migration in vitro (Laskin, 1986; Senior, 1989) and neutrophil accumulation in the lungs of rats following intratracheal instillation (Riley, 1988). In addition, in studies of alkali-induced corneal injury in rabbits, a neutrophilic chemotactic activity was found (Pfister, 1988), which was later shown to be a collagen-derived tripeptide, PGP (Pfister, 1995). This tripeptide was generated in two forms, N-acetyl-PGP and N-methyl-PGP, but the acetylated form proved to be significantly more active as a neutrophil chemoattractant (Haddox, 1999). MMP-9 may be one of the proteases involved in the generation of PGP in vivo judging from a recent study of bacterial infection in mice. MMP-9-deficient mice showed less PGP and fewer neutrophils in bronchoalveolar lavage fluid, and better preservation of collagen fibrils in the lungs, than controls in a model of F. tularensis pulmonary infection (Malik, 2007). Prolyl endopeptidase, a serine endoprotease with the ability to cleave peptide bonds on the carboxyl side of proline residues in peptides shorter than 30 amino acids, appears to act in concert with MMP-8 and/or MMP-9 to generate PGP from collagen (J. Edwin Blalock, University of Alabama, personal communication). The in vivo mechanism of acetylation of PGP is still unknown.
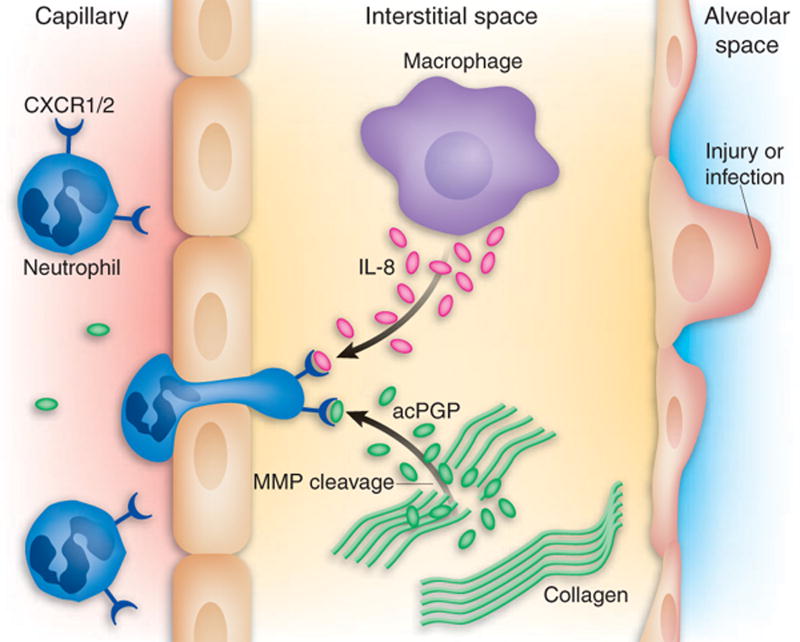
In the study by Weathington et. al. discussed in section 2.1 above, LPS-instillation into the lungs of mice generated acPGP and neutrophil accumulation. By administering a monoclonal PGP-blocking antibody concurrent with LPS, the neutrophil accumulation in response to LPS was dramatically reduced. Further, intrapulmonary instillation of acPGP promoted neutrophil recruitment in the lungs of wild-type mice, but not in mice lacking CXCR2, the receptor for the mouse equivalent of IL-8 (Fig. 3). These data, along with in vitro data using human neutrophils and CXCR1 and CXCR2 blocking antibodies, indicated that PGP's mode of action involves binding to CXCR2 on murine neutrophils, and CXCR1 and CXCR2 on human neutrophils. Repeated intratracheal instillation of PGP into mice caused airspace enlargement resembling emphysema. Also, in this study PGP was found in bronchoalveolar lavage fluid from individuals with chronic obstructive pulmonary disease, a condition in which there is chronic inflammation of lung tissue.
Fig. 3. Chemotactic activity of a collagen-derived fragment.
Early in an inflammatory response, human neutrophils react to CXC chemokines, such as IL-8, by following the chemotactic gradient and emigrating into the interstitial space. This chemotactic response depends upon the ability of IL-8 to engage either of its two receptors, CXCR1 and CXCR2. Proteolytic cleavage of type I collagen, presumably by MMPs and subsequent acetylation, generates an acetylated pro-gly-pro, which mimics a key motif of IL-8, and thus stimulates CXCR1 and CXCR2, prolonging the influx of neutrophils. Taken from (Henson, 2006) with permission.
3.4 Hyaluronan fragments induce cytokines
Hyaluronan is a polymeric component of the ECM consisting of repeating disaccharide units of D-glucuronic acid and N-acetyl glucosamine. Under normal conditions it is present in a high molecular weight form (>1000 kDa). In tissue injury and inflammation hyaluronan can undergo processing to lower molecular weight forms that induce the expression of a variety of genes involved in inflammation in various cells types, including macrophages. Initial studies indicated that signaling from hyaluronan degradation products involves CD44 exclusively. However, studies of CD44 null macrophages indicate that there are other signaling pathways, notably through Toll-like receptors, TLR2 and TLR4 (Jiang, 2005). Hyaluronan degradation products are likely to be relevant in vivo as circulating levels are detectable in individuals with acute lung injury and when these products are isolated they induce murine macrophages to express cytokines such as MIP-1α, MIP-1β, MCP-1, KC, IL-8, and IL-12 (McKee, 1996; Hodge-Dufour, 1997).
4. Summary
ECM components have domains that interact with receptors on inflammatory cells. Thus, exposure or release of these matrix-derived domains from intact ECM molecules, as may occur by proteolytic activity during inflammation and tissue injury, can lead to a variety of inflammatory cell responses. Recent studies clearly show that bioactive domains of ECM can be generated in vivo. Receptors for active domains in several ECM components have been determined at the molecular level and the intracellular signaling pathways triggered by receptors for ECM-derived ligands are under study.
5. Conclusion
In addition to its classical role of providing structural support for cells and tissues, fragments released from many ECM components trigger various responses in inflammatory and immune cells.
Acknowledgments
This work was funded by the Francis Family Foundation (T.L.A.-K.), the Alan A. and Edith L. Wolf Charitable Trust / Barnes-Jewish Hospital Foundation (R.M.S.), and NHLBI/NIH P01 HL 29594 (R.M.S.).
Abbreviations
- ECM
extracellular matrix
- EBP
elastin binding protein
- acPGP
N-acetylated Pro-Gly-Pro
- MMP
matrix metalloproteinase
- SIKVAV
Ser-Ile-Lys-Val-Ala-Val
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adair-Kirk TL, Atkinson JJ, Broekelmann TJ, Doi M, Tryggvason K, Miner JH, Mecham RP, Senior RM. A site on laminin alpha 5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J Immunol. 2003;171:398–406. doi: 10.4049/jimmunol.171.1.398. [DOI] [PubMed] [Google Scholar]
- Adair-Kirk TL, Atkinson JJ, Kelley DG, Arch RH, Miner JH, Senior RM. A chemotactic peptide from laminin alpha 5 functions as a regulator of inflammatory immune responses via TNF alpha-mediated signaling. J Immunol. 2005;174:1621–1629. doi: 10.4049/jimmunol.174.3.1621. [DOI] [PubMed] [Google Scholar]
- Alon R, Cahalon L, Hershkoviz R, Elbaz D, Reizis B, Wallach D, Akiyama SK, Yamada KM, Lider O. TNF-alpha binds to the N-terminal domain of fibronectin and augments the beta 1-integrin-mediated adhesion of CD4+ T lymphocytes to the glycoprotein. J Immunol. 1994;152:1304–1313. [PubMed] [Google Scholar]
- Andersson E, Rydengard V, Sonesson A, Morgelin M, Bjorck L, Schmidtchen A. Antimicrobial activities of heparin-binding peptides. Eur J Biochem. 2004;271:1219–1226. doi: 10.1111/j.1432-1033.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- Baranek T, Debret R, Antonicelli F, Lamkhioued B, Belaaouaj A, Hornebeck W, Bernard P, Guenounou M, Le Naour R. Elastin receptor (spliced galactosidase) occupancy by elastin peptides counteracts proinflammatory cytokine expression in lipopolysaccharide-stimulated human monocytes through NF-kappaB down-regulation. J Immunol. 2007;179:6184–6192. doi: 10.4049/jimmunol.179.9.6184. [DOI] [PubMed] [Google Scholar]
- Beezhold DH, Personius C. Fibronectin fragments stimulate tumor necrosis factor secretion by human monocytes. J Leukoc Biol. 1992;51:59–64. doi: 10.1002/jlb.51.1.59. [DOI] [PubMed] [Google Scholar]
- Bohnsack JF, Kleinman HK, Takahashi T, O'Shea JJ, Brown EJ. Connective tissue proteins and phagocytic cell function. Laminin enhances complement and Fc-mediated phagocytosis by cultured human macrophages. J Exp Med. 1985;161:912–923. doi: 10.1084/jem.161.5.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ. The role of extracellular matrix proteins in the control of phagocytosis. J Leukoc Biol. 1986;39:579–591. doi: 10.1002/jlb.39.5.579. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Goodwin JL. Fibronectin receptors of phagocytes. Characterization of the Arg-Gly-Asp binding proteins of human monocytes and polymorphonuclear leukocytes. J Exp Med. 1988;167:777–793. doi: 10.1084/jem.167.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran ML, Kibbey MC, Kleinman HK, Wahl LM. Laminin SIKVAV peptide induction of monocyte/macrophage prostaglandin E2 and matrix metalloproteinases. J Biol Chem. 1995;270:10365–10368. doi: 10.1074/jbc.270.18.10365. [DOI] [PubMed] [Google Scholar]
- Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debret R, Antonicelli F, Theill A, Hornebeck W, Bernard P, Guenounou M, Le Naour R. Elastin-derived peptides induce a T-helper type 1 polarization of human blood lymphocytes. Arterioscler Thromb Vasc Biol. 2005;25:1353–1358. doi: 10.1161/01.ATV.0000168412.50855.9f. [DOI] [PubMed] [Google Scholar]
- Debret R, Le Naour RR, Sallenave JM, Deshorgue A, Hornebeck WG, Guenounou M, Bernard P, Antonicelli FD. Elastin fragments induce IL-1beta upregulation via NF-kappaB pathway in melanoma cells. J Invest Dermatol. 2006;126:1860–1868. doi: 10.1038/sj.jid.5700337. [DOI] [PubMed] [Google Scholar]
- Duca L, Floquet N, Alix AJ, Haye B, Debelle L. Elastin as a matrikine. Crit Rev Oncol Hematol. 2004;49:235–244. doi: 10.1016/j.critrevonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Duca L, Blanchevoye C, Cantarelli B, Ghoneim C, Dedieu S, Delacoux F, Hornebeck W, Hinek A, Martiny L, Debelle L. The elastin receptor complex transduces signals through the catalytic activity of its Neu-1 subunit. J Biol Chem. 2007;282:12484–12491. doi: 10.1074/jbc.M609505200. [DOI] [PubMed] [Google Scholar]
- Faisal Khan KM, Laurie GW, McCaffrey TA, Falcone DJ. Exposure of cryptic domains in the alpha 1-chain of laminin-1 by elastase stimulates macrophages urokinase and matrix metalloproteinase-9 expression. J Biol Chem. 2002;277:13778–13786. doi: 10.1074/jbc.M111290200. [DOI] [PubMed] [Google Scholar]
- Freitas VM, Vilas-Boas VF, Pimenta DC, Loureiro V, Juliano MA, Carvalho MR, Pinheiro JJ, Camargo AC, Moriscot AS, Hoffman MP, Jaeger RG. SIKVAV, a laminin alpha1-derived peptide, interacts with integrins and increases protease activity of a human salivary gland adenoid cystic carcinoma cell line through the ERK 1/2 signaling pathway. Am J Pathol. 2007;171:124–138. doi: 10.2353/ajpath.2007.051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Booms P, Halushka M, Dietz HC, Ney A, Stricker S, Hecht J, Mundlos S, Robinson PN. Induction of macrophage chemotaxis by aortic extracts of the mgR Marfan mouse model and a GxxPG-containing fibrillin-1 fragment. Circulation. 2006;114:1855–1862. doi: 10.1161/CIRCULATIONAHA.105.601674. [DOI] [PubMed] [Google Scholar]
- Haddox JL, Pfister RR, Muccio DD, Villain M, Sommers CI, Chaddha M, Anantharamaiah GM, Brouillette WJ, DeLucas LJ. Bioactivity of peptide analogs of the neutrophil chemoattractant, N-acetyl-proline-glycine-proline. Invest Ophthalmol Vis Sci. 1999;40:2427–2429. [PubMed] [Google Scholar]
- Hance KA, Tataria M, Ziporin SJ, Lee JK, Thompson RW. Monocyte chemotactic activity in human abdominal aortic aneurysms: role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J Vasc Surg. 2002;35:254–261. doi: 10.1067/mva.2002.120382. [DOI] [PubMed] [Google Scholar]
- Hermann M, Jaconi ME, Dahlgren C, Waldvogel FA, Stendahl O, Lew DP. Neutrophil bactericidal activity against Staphylococcus aureus adherent on biological surfaces. Surface-bound extracellular matrix proteins activate intracellular killing by oxygen-dependent and -independent mechanisms. J Clin Invest. 1990;86:942–951. doi: 10.1172/JCI114796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkoviz R, Cahalon L, Miron S, Alon R, Sapir T, Akiyama SK, Yamada KM, Lider O. TNF-alpha associated with fibronectin enhances phorbol myristate acetate- or antigen-mediated integrin-dependent adhesion of CD4+ T cells via protein tyrosine phosphorylation. J Immunol. 1994;153:554–565. [PubMed] [Google Scholar]
- Hershkoviz R, Goldkorn I, Lider O. Tumour necrosis factor-alpha interacts with laminin and functions as a pro-adhesive cytokine. Immunology. 1995;85:125–130. [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Wrenn DS, Mecham RP, Barondes SH. The elastin receptor: a galactoside-binding protein. Science. 1988;239:1539–1541. doi: 10.1126/science.2832941. [DOI] [PubMed] [Google Scholar]
- Hinek A. Biological roles of the non-integrin elastin/laminin receptor. Biol Chem. 1996;377:471–480. [PubMed] [Google Scholar]
- Hodge-Dufour J, Noble PW, Horton MR, Bao C, Wysoka M, Burdick MD, Strieter RM, Trinchieri G, Pure E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol. 1997;159:2492–2500. [PubMed] [Google Scholar]
- Hornebeck W, Maquart FX. Proteolyzed matrix as a template for the regulation of tumor progression. Biomed Pharmacother. 2003;57:223–230. doi: 10.1016/s0753-3322(03)00049-0. [DOI] [PubMed] [Google Scholar]
- Horton MR, Burdick MD, Strieter RM, Bao C, Noble PW. Regulation of hyaluronan-induced chemokine gene expression by IL-10 and IFN-gamma in mouse macrophages. J Immunol. 1998a;160:3023–3030. [PubMed] [Google Scholar]
- Horton MR, McKee CM, Bao C, Liao F, Farber JM, Hodge-DuFour J, Pure E, Oliver BL, Wright TM, Noble PW. Hyaluronan fragments synergize with interferon-gamma to induce the C-X-C chemokines mig and interferon-inducible protein-10 in mouse macrophages. J Biol Chem. 1998b;273:35088–35094. doi: 10.1074/jbc.273.52.35088. [DOI] [PubMed] [Google Scholar]
- Horton MR, Shapiro S, Bao C, Lowenstein CJ, Noble PW. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J Immunol. 1999;162:4171–4176. [PubMed] [Google Scholar]
- Horton MR, Olman MA, Bao C, White KE, Choi AM, Chin BY, Noble PW, Lowenstein CJ. Regulation of plasminogen activator inhibitor-1 and urokinase by hyaluronan fragments in mouse macrophages. Am J Physiol Lung Cell Mol Physiol. 2000;279:L707–715. doi: 10.1152/ajplung.2000.279.4.L707. [DOI] [PubMed] [Google Scholar]
- Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi K, Nagaoka I, Someya A, Yamashita T. Type IV collagen-binding proteins of neutrophils: possible involvement of L-selectin in the neutrophil binding to type IV collagen. Blood. 1996;87:365–372. [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Khan KM, Falcone DJ. Role of laminin in matrix induction of macrophage urokinase-type plasminogen activator and 92-kDa metalloproteinase expression. J Biol Chem. 1997;272:8270–8275. doi: 10.1074/jbc.272.13.8270. [DOI] [PubMed] [Google Scholar]
- Khan KM, Falcone DJ. Selective activation of MAPK(erk1/2) by laminin-1 peptide alpha1:Ser(2091)-Arg(2108) regulates macrophage degradative phenotype. J Biol Chem. 2000;275:4492–4498. doi: 10.1074/jbc.275.6.4492. [DOI] [PubMed] [Google Scholar]
- Kreikemeyer B, Klenk M, Podbielski A. The intracellular status of Streptococcus pyogenes: role of extracellular matrix-binding proteins and their regulation. Int J Med Microbiol. 2004;294:177–188. doi: 10.1016/j.ijmm.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Labat-Robert J, Robert L. Introduction: matrix biology in the 21st century. From a static-rheological role to a dynamic-signaling function. Pathol Biol (Paris) 2005;53:369–371. doi: 10.1016/j.patbio.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Labat-Robert J, Robert L. The effect of cell-matrix interactions and aging on the malignant process. Adv Cancer Res. 2007;98:221–259. doi: 10.1016/S0065-230X(06)98007-5. [DOI] [PubMed] [Google Scholar]
- Larbi A, Levesque G, Robert L, Gagne D, Douziech N, Fulop T., Jr Presence and active synthesis of the 67 kDa elastin-receptor in human circulating white blood cells. Biochem Biophys Res Commun. 2005;332:787–792. doi: 10.1016/j.bbrc.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Kimura T, Sakakibara S, Riley DJ, Berg RA. Chemotactic activity of collagen-like polypeptides for human peripheral blood neutrophils. J Leukoc Biol. 1986;39:255–266. doi: 10.1002/jlb.39.3.255. [DOI] [PubMed] [Google Scholar]
- Laurent F, Benoliel AM, Capo C, Bongrand P. Oxidative metabolism of polymorphonuclear leukocytes: modulation by adhesive stimuli. J Leukoc Biol. 1991;49:217–226. doi: 10.1002/jlb.49.3.217. [DOI] [PubMed] [Google Scholar]
- Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Cogswell S, Storness-Bliss C, Corry DB, Kheradmand F. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- Lopez-Moratalla N, del Mar Calonge M, Lopez-Zabalza MJ, Perez-Mediavilla LA, subira ML, Santiago E. Activation of human lymphomononuclear cells by peptides derived from extracellular matrix proteins. Biochim Biophys Acta. 1995;1265:181–188. doi: 10.1016/0167-4889(94)00199-o. [DOI] [PubMed] [Google Scholar]
- Malik M, Bakshi CS, McCabe K, Catlett SV, Shah A, Singh R, Jackson PL, Gaggar A, Metzger DW, Melendez JA, Blalock JE, Sellati TJ. Matrix metalloproteinase 9 activity enhances host susceptibility to pulmonary infection with type A and B strains of Francisella tularensis. J Immunol. 2007;178:1013–1020. doi: 10.4049/jimmunol.178.2.1013. [DOI] [PubMed] [Google Scholar]
- Malmsten M, Davoudi M, Schmidtchen A. Bacterial killing by heparin-binding peptides from PRELP and thrombospondin. Matrix Biol. 2006;25:294–300. doi: 10.1016/j.matbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Malvagia S, Morrone A, Caciotti A, Bardelli T, d'Azzo A, Ancora G, Zammarchi E, Donati MA. New mutations in the PPBG gene lead to loss of PPCA protein which affects the level of the beta-galactosidase/neuraminidase complex and the EBP-receptor. Mol Genet Metab. 2004;82:48–55. doi: 10.1016/j.ymgme.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Maquart FX, Bellon G, Pasco S, Monboisse JC. Matrikines in the regulation of extracellular matrix degradation. Biochimie. 2005;87:353–360. doi: 10.1016/j.biochi.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Maquart FX, Pasco S, Ramont L, Hornebeck W, Monboisse JC. An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit Rev Oncol Hematol. 2004;49:199–202. doi: 10.1016/j.critrevonc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- Marom B, Rahat MA, Lahat N, Weiss-Cerem L, Kinarty A, Bitterman H. Native and fragmented fibronectin oppositely modulate monocyte secretion of MMP-9. J Leukoc Biol. 2007;81:1466–1476. doi: 10.1189/jlb.0506328. [DOI] [PubMed] [Google Scholar]
- McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monboisse JC, Bellon G, Dufer J, Randoux A, Borel JP. Collagen activates superoxide anion production by human polymorphonuclear neutrophils. Biochem J. 1987;246:599–603. doi: 10.1042/bj2460599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monboisse JC, Garnotel R, Bellon G, Ohno N, Perreau C, Borel JP, Kefalides NA. The alpha 3 chain of type IV collagen prevents activation of human polymorphonuclear leukocytes. J Biol Chem. 1994;269:25475–25482. [PubMed] [Google Scholar]
- Ottonello L, Dapino P, Amelotti M, Barbera P, Arduino N, Bertolotto M, Dallegri F. Activation of neutrophil respiratory burst by cytokines and chemoattractants. Regulatory role of extracellular matrix glycoproteins. Inflamm Res. 1998;47:345–350. doi: 10.1007/s000110050340. [DOI] [PubMed] [Google Scholar]
- Parker CJ, Frame RN, Elstad MR. Vitronectin (S protein) augments the functional activity of monocyte receptors for IgG and complement C3b. Blood. 1988;71:86–93. [PubMed] [Google Scholar]
- Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- Pfister RR, Haddox JL, Lam KW, Lank KM. Preliminary characterization of a polymorphonuclear leukocyte stimulant isolated from alkali-treated collagen. Invest Ophthalmol Vis Sci. 1988;29:955–962. [PubMed] [Google Scholar]
- Pfister RR, Haddox JL, Sommers CI, Lam KW. Identification and synthesis of chemotactic tripeptides from alkali-degraded whole cornea. A study of N-acetyl-proline-glycine-proline and N-methyl-proline-glycine-proline. Invest Ophthalmol Vis Sci. 1995;36:1306–1316. [PubMed] [Google Scholar]
- Pike MC, Wicha MS, Yoon P, Mayo L, Boxer LA. Laminin promotes the oxidative burst in human neutrophils via increased chemoattractant receptor expression. J Immunol. 1989;142:2004–2011. [PubMed] [Google Scholar]
- Privitera S, Prody CA, Callahan JW, Hinek A. The 67-kDa enzymatically inactive alternatively spliced variant of beta-galactosidase is identical to the elastin/laminin-binding protein. J Biol Chem. 1998;273:6319–6326. doi: 10.1074/jbc.273.11.6319. [DOI] [PubMed] [Google Scholar]
- Riley DJ, Berg RA, Soltys RA, Kerr JS, Guss HN, Curran SF, Laskin DL. Neutrophil response following intratracheal instillation of collagen peptides into rat lungs. Exp Lung Res. 1988;14:549–563. doi: 10.3109/01902148809087827. [DOI] [PubMed] [Google Scholar]
- Schenk S, Quaranta V. Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol. 2003;13:366–375. doi: 10.1016/s0962-8924(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Senior RM, Skogen WF, Griffin GL, Wilner GD. Effects of fibrinogen derivatives upon the inflammatory response. Studies with human fibrinopeptide B. J Clin Invest. 1986;77:1014–1019. doi: 10.1172/JCI112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior RM, Hinek A, Griffin GL, Pipoly DJ, Crouch EC, Mecham RP. Neutrophils show chemotaxis to type IV collagen and its 7S domain and contain a 67 kD type IV collagen binding protein with lectin properties. Am J Respir Cell Mol Biol. 1989;1:479–487. doi: 10.1165/ajrcmb/1.6.479. [DOI] [PubMed] [Google Scholar]
- Senior RM, Gresham HD, Griffin GL, Brown EJ, Chung AE. Entactin stimulates neutrophil adhesion and chemotaxis through interactions between its Arg-Gly-Asp (RGD) domain and the leukocyte response integrin. J Clin Invest. 1992;90:2251–2257. doi: 10.1172/JCI116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms HH, D'Amico R. Studies on polymorphonuclear leukocyte bactericidal function III: the role of extracellular matrix proteins. J Surg Res. 1997;72:123–128. doi: 10.1006/jsre.1997.5184. [DOI] [PubMed] [Google Scholar]
- Steadman R, Irwin MH, John PL, Blackburn WD, Heck LW, Abrahamson DR. Laminin cleavage by activated human neutrophils yields proteolytic fragments with selective migratory properties. J Leukoc Biol. 1993;53:354–365. doi: 10.1002/jlb.53.4.354. [DOI] [PubMed] [Google Scholar]
- Tatano Y, Takeuchi N, Kuwahara J, Sakuraba H, Takahashi T, Takada G, Itoh K. Elastogenesis in cultured dermal fibroblasts from patients with lysosomal beta-galactosidase, protective protein/cathepsin A and neuraminidase-1 deficiencies. J Med Invest. 2006;53:103–112. doi: 10.2152/jmi.53.103. [DOI] [PubMed] [Google Scholar]
- Tran KT, Griffith L, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004;12:262–268. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- Vaday GG, Franitza S, Schor H, Hecht I, Brill A, Cahalon L, Hershkoviz R, Lider O. Combinatorial signals by inflammatory cytokines and chemokines mediate leukocyte interactions with extracellular matrix. J Leukoc Biol. 2001;69:885–892. [PubMed] [Google Scholar]
- Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- Xie DL, Meyers R, Homandberg GA. Release of elastase from monocytes adherent to a fibronectin-gelatin surface. Blood. 1993;81:186–192. [PubMed] [Google Scholar]
- Yang KD, Augustine NH, Shaio MF, Bohnsack JF, Hill HR. Effects of fibronectin on actin organization and respiratory burst activity in neutrophils, monocytes, and macrophages. J Cell Physiol. 1994;158:347–353. doi: 10.1002/jcp.1041580217. [DOI] [PubMed] [Google Scholar]
- Yonemasu K, Sasaki T, Hashimoto H, Kashiba S. Opsonic effect of fibronectin on staphylococcal phagocytosis by human polymorphonuclear leukocytes: its relative inefficiency in post-phagocytic metabolic activities and in intracellular killing. Microbiol Immunol. 1988;32:795–805. doi: 10.1111/j.1348-0421.1988.tb01441.x. [DOI] [PubMed] [Google Scholar]