Abstract
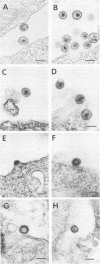
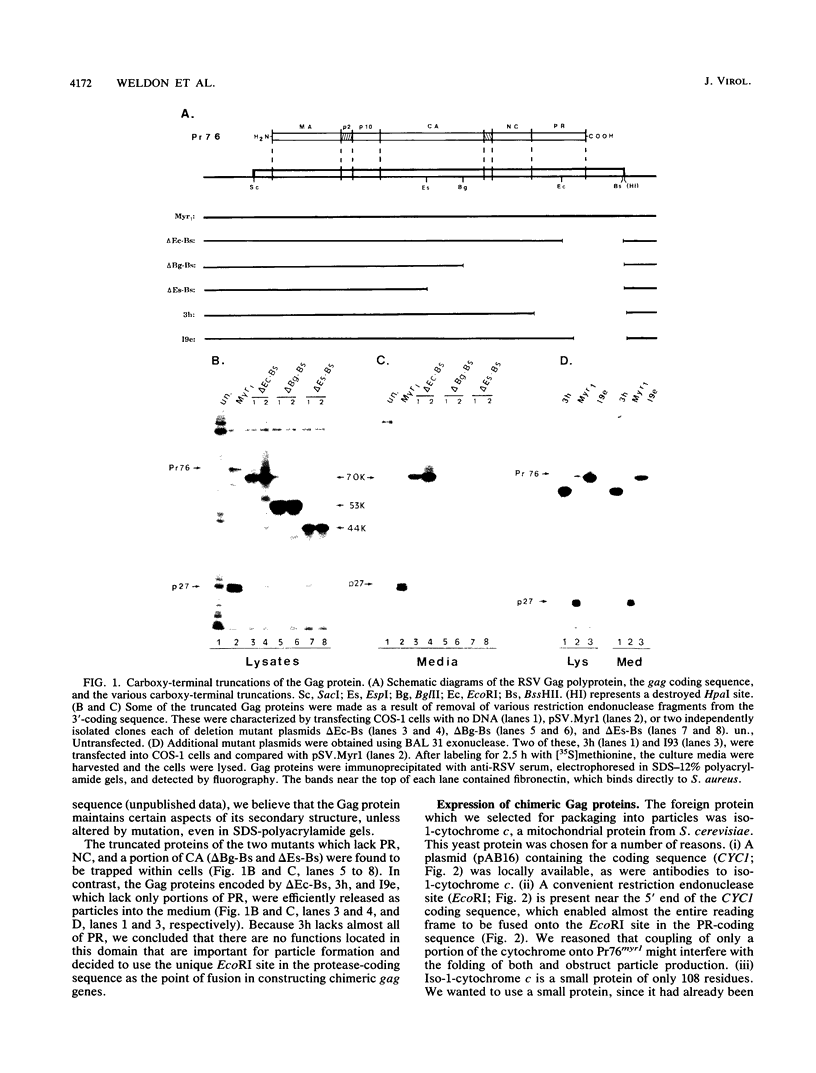
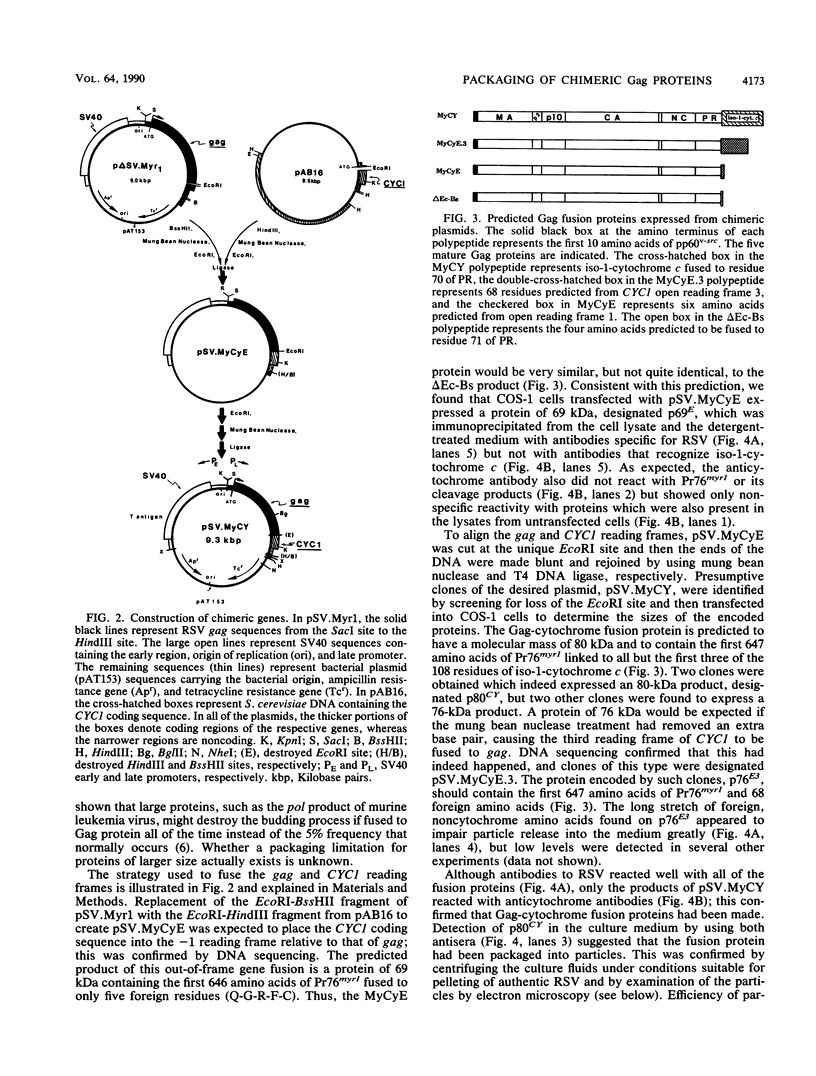
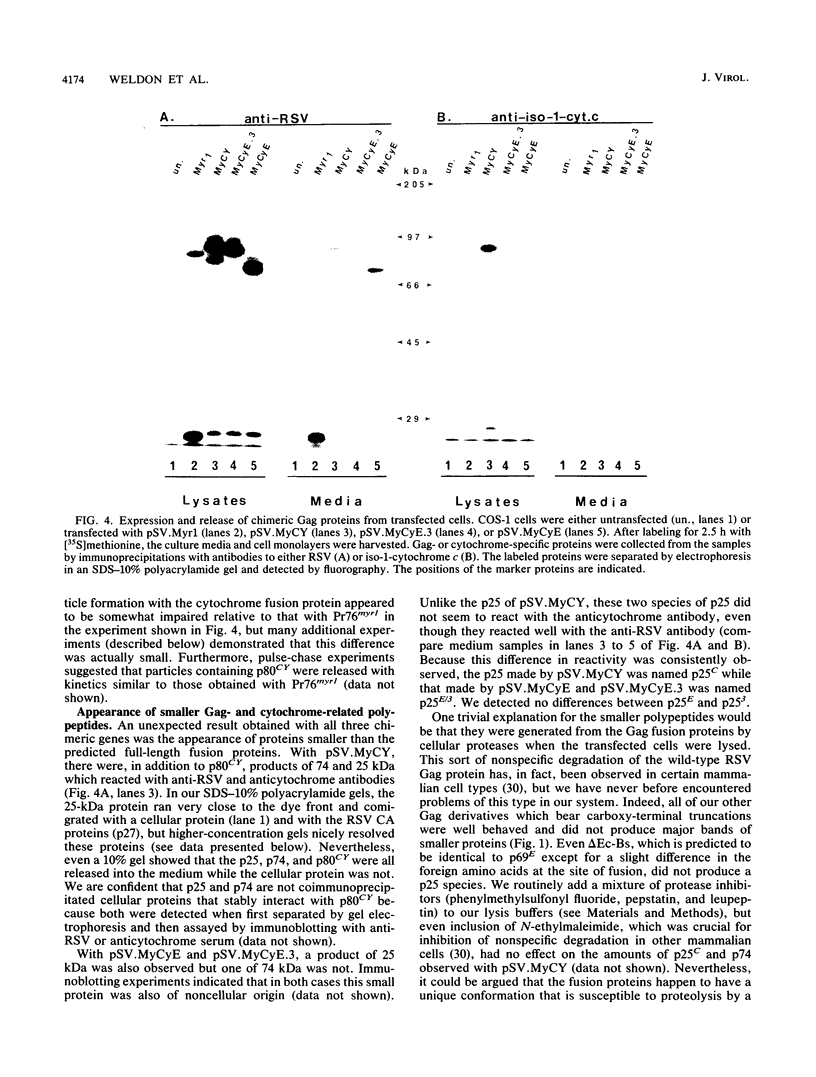
The product of the Rous sarcoma virus (RSV) gag gene, Pr76gag, is a polyprotein precursor which is cleaved by the viral protease to yield the major structural proteins of the virion during particle assembly in avian host cells. We have recently shown that myristylated forms of the RSV Gag protein can induce particle formation with very high efficiency when expressed in mammalian cells (J. W. Wills, R. C. Craven, and J. A. Achacoso, J. Virol. 63:4331-4343, 1989). We made use of this mammalian system to examine the abilities of foreign antigens to be incorporated into particles when fused directly to the myristylated Gag protein. Our initial experiments showed that removal of various portions of the viral protease located at the carboxy terminus of the RSV Gag protein did not disrupt particle formation. We therefore chose this region for coupling of iso-1-cytochrome c from Saccharomyces cerevisiae to Gag. This was accomplished by constructing an in-frame fusion of the CYC1 and gag coding sequences at a common restriction endonuclease site. Expression of the chimeric gene resulted in synthesis of the Gag-cytochrome fusion protein and its release into the cell culture medium. The chimeric particles were readily purified by simple centrifugation, and transmission electron microscopy of cells that produced them revealed a morphology similar to that of immature type C retrovirions.
Full text
PDF




Images in this article
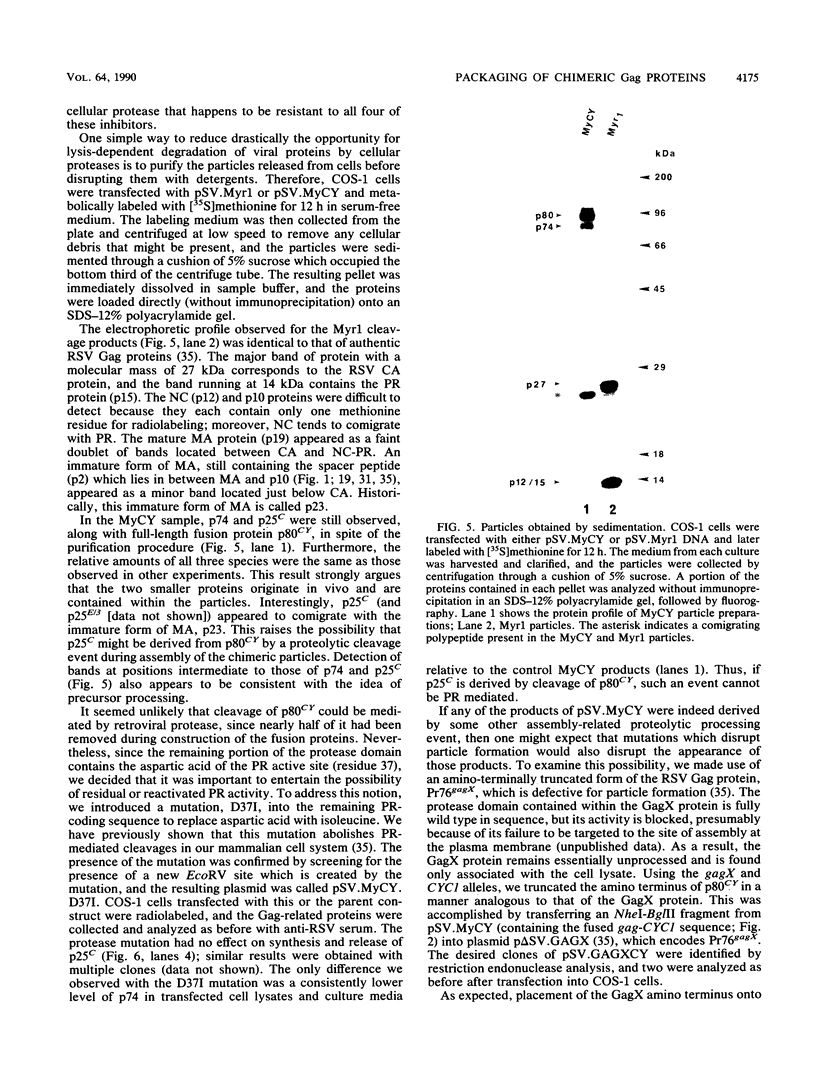
Selected References
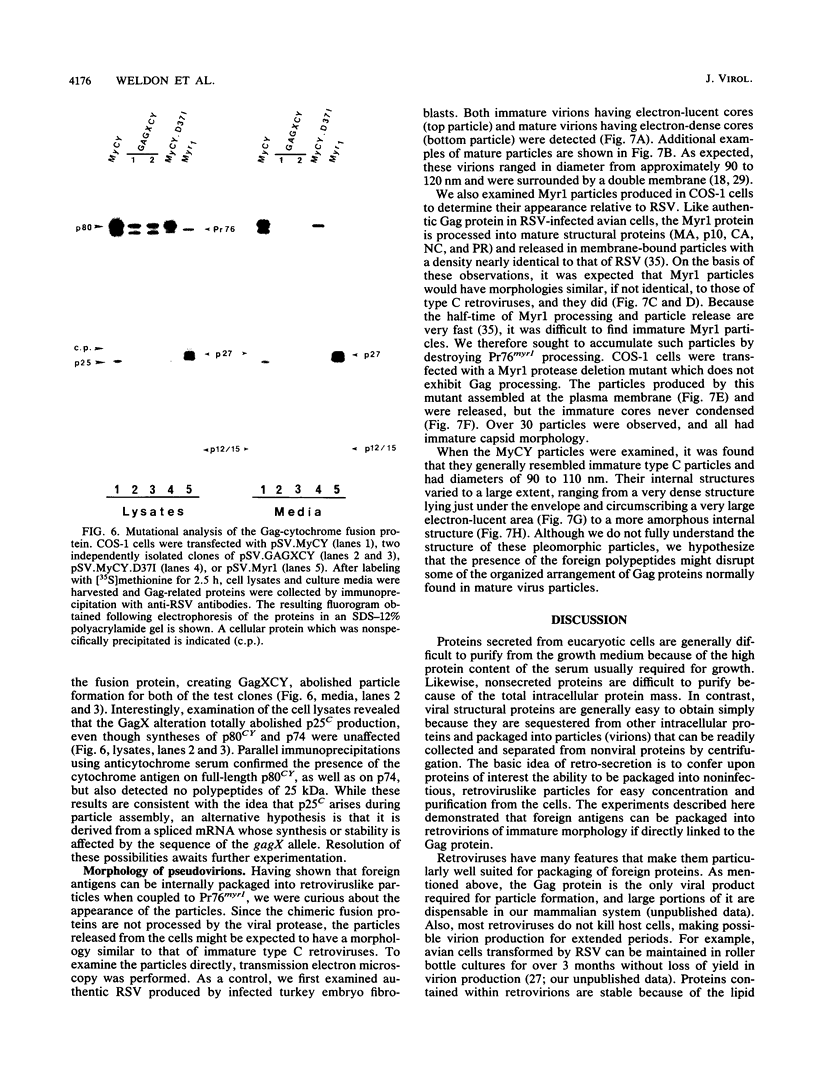
These references are in PubMed. This may not be the complete list of references from this article.
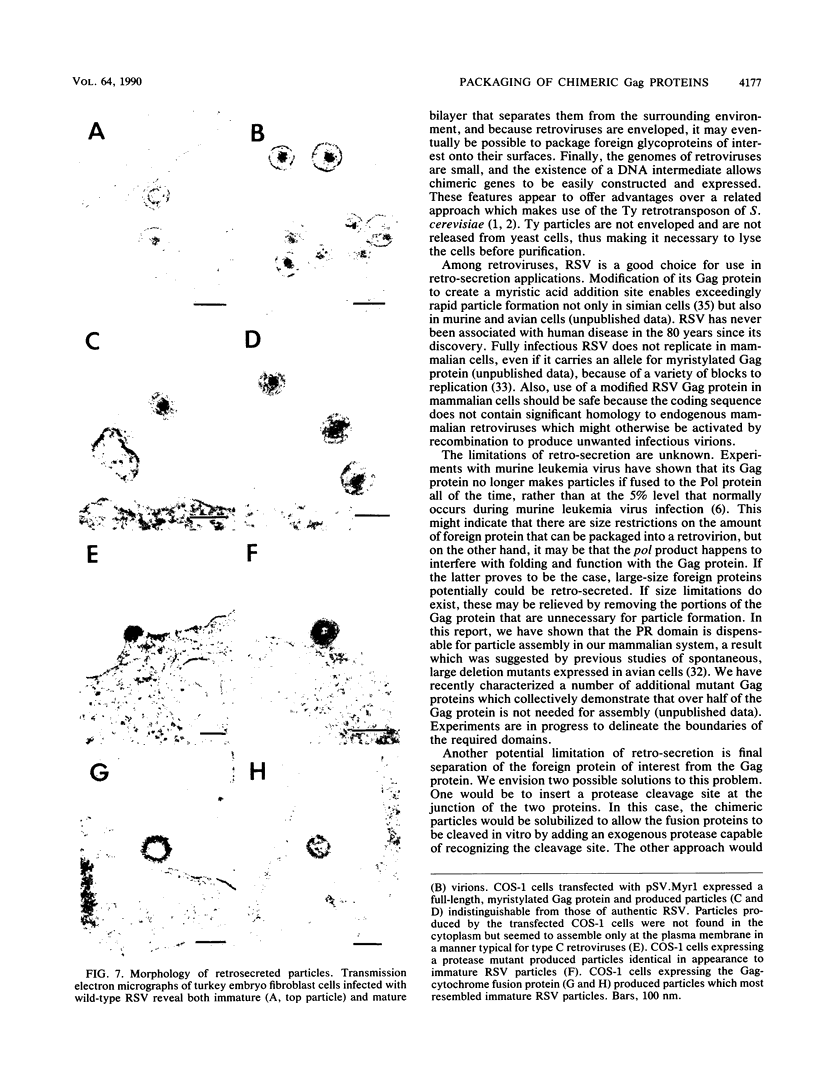
- Adams S. E., Dawson K. M., Gull K., Kingsman S. M., Kingsman A. J. The expression of hybrid HIV:Ty virus-like particles in yeast. Nature. 1987 Sep 3;329(6134):68–70. doi: 10.1038/329068a0. [DOI] [PubMed] [Google Scholar]
- Adams S. E., Mellor J., Gull K., Sim R. B., Tuite M. F., Kingsman S. M., Kingsman A. J. The functions and relationships of Ty-VLP proteins in yeast reflect those of mammalian retroviral proteins. Cell. 1987 Apr 10;49(1):111–119. doi: 10.1016/0092-8674(87)90761-6. [DOI] [PubMed] [Google Scholar]
- Delchambre M., Gheysen D., Thines D., Thiriart C., Jacobs E., Verdin E., Horth M., Burny A., Bex F. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989 Sep;8(9):2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein K. M., Goff S. P. Expression of the gag-pol fusion protein of Moloney murine leukemia virus without gag protein does not induce virion formation or proteolytic processing. J Virol. 1988 Jun;62(6):2179–2182. doi: 10.1128/jvi.62.6.2179-2182.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis B., Linial M., Eisenman R. An avian oncovirus mutant deficient in genomic RNA: characterization of the packaged RNA as cellular messenger RNA. Virology. 1979 Apr 15;94(1):146–161. doi: 10.1016/0042-6822(79)90445-8. [DOI] [PubMed] [Google Scholar]
- Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D., De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989 Oct 6;59(1):103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P. Characterization of a replication-defective temperature-sensitive mutant of Rous sarcoma virus. Virology. 1984 Jun;135(2):480–488. doi: 10.1016/0042-6822(84)90202-2. [DOI] [PubMed] [Google Scholar]
- Katoh I., Ikawa Y., Yoshinaka Y. Retrovirus protease characterized as a dimeric aspartic proteinase. J Virol. 1989 May;63(5):2226–2232. doi: 10.1128/jvi.63.5.2226-2232.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Rein A., Shibuya M., Odaka T., Oroszlan S. Murine leukemia virus maturation: protease region required for conversion from "immature" to "mature" core form and for virus infectivity. Virology. 1985 Sep;145(2):280–292. doi: 10.1016/0042-6822(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Leis J., Baltimore D., Bishop J. M., Coffin J., Fleissner E., Goff S. P., Oroszlan S., Robinson H., Skalka A. M., Temin H. M. Standardized and simplified nomenclature for proteins common to all retroviruses. J Virol. 1988 May;62(5):1808–1809. doi: 10.1128/jvi.62.5.1808-1809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Medeiros E., Hayward W. S. An avian oncovirus mutant (SE 21Q1b) deficient in genomic RNA: biological and biochemical characterization. Cell. 1978 Dec;15(4):1371–1381. doi: 10.1016/0092-8674(78)90062-4. [DOI] [PubMed] [Google Scholar]
- Miller M., Jaskólski M., Rao J. K., Leis J., Wlodawer A. Crystal structure of a retroviral protease proves relationship to aspartic protease family. Nature. 1989 Feb 9;337(6207):576–579. doi: 10.1038/337576a0. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Fine-structure analyses of lipid-protein and protein-protein interactions of gag protein p19 of the avian sarcoma and leukemia viruses by cyanogen bromide mapping. J Virol. 1984 Oct;52(1):145–153. doi: 10.1128/jvi.52.1.145-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro J. H., Ghosh H. P. Impaired cleavage of the joint gag-pol polyprotein precursor and virion assembly in a temperature-sensitive mutant of Rous sarcoma virus. Virology. 1984 Jun;135(2):489–502. doi: 10.1016/0042-6822(84)90203-4. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Linial M. Avian oncovirus mutant (SE21Q1b) deficient in genomic RNA: characterization of a deletion in the provirus. J Virol. 1980 Nov;36(2):450–456. doi: 10.1128/jvi.36.2.450-456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Garoff H. The budding mechanisms of enveloped animal viruses. J Gen Virol. 1980 Sep;50(1):1–21. doi: 10.1099/0022-1317-50-1-1. [DOI] [PubMed] [Google Scholar]
- Skalka A. M. Retroviral proteases: first glimpses at the anatomy of a processing machine. Cell. 1989 Mar 24;56(6):911–913. doi: 10.1016/0092-8674(89)90621-1. [DOI] [PubMed] [Google Scholar]
- Smith M., Leung D. W., Gillam S., Astell C. R., Montgomery D. L., Hall B. D. Sequence of the gene for iso-1-cytochrome c in Saccharomyces cerevisiae. Cell. 1979 Apr;16(4):753–761. doi: 10.1016/0092-8674(79)90091-6. [DOI] [PubMed] [Google Scholar]
- Smith R. E. Large-scale growth of Rous sarcoma virus. Methods Enzymol. 1979;58:393–403. doi: 10.1016/s0076-6879(79)58154-3. [DOI] [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W. Assembly of animal viruses at cellular membranes. Annu Rev Microbiol. 1988;42:489–516. doi: 10.1146/annurev.mi.42.100188.002421. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Bruckenstein D. A., Bell A. P. Avian sarcoma virus gag precursor polypeptide is not processed in mammalian cells. J Virol. 1982 Nov;44(2):725–730. doi: 10.1128/jvi.44.2.725-730.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Pepinsky R. B., Southard L. E. Primary structure of p19 species of avian sarcoma and leukemia viruses. J Virol. 1985 Oct;56(1):31–39. doi: 10.1128/jvi.56.1.31-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow S. L., Coffin J. M. Truncated gag-related proteins are produced by large deletion mutants of Rous sarcoma virus and form virus particles. J Virol. 1985 Jul;55(1):79–85. doi: 10.1128/jvi.55.1.79-85.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Achacoso J. A. Creation and expression of myristylated forms of Rous sarcoma virus gag protein in mammalian cells. J Virol. 1989 Oct;63(10):4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W. Retro-secretion of recombinant proteins. Nature. 1989 Jul 27;340(6231):323–324. doi: 10.1038/340323a0. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Srinivas R. V., Hunter E. Mutations of the Rous sarcoma virus env gene that affect the transport and subcellular location of the glycoprotein products. J Cell Biol. 1984 Dec;99(6):2011–2023. doi: 10.1083/jcb.99.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Ishigame K., Ohno T., Kageyama S., Shibata K., Luftig R. B. Preparations enriched for "immature" murine leukemia virus particles that remain in tissue culture fluids are deficient in Pr65gag proteolyic activity. Virology. 1980 Jan 15;100(1):130–140. doi: 10.1016/0042-6822(80)90559-0. [DOI] [PubMed] [Google Scholar]