Abstract
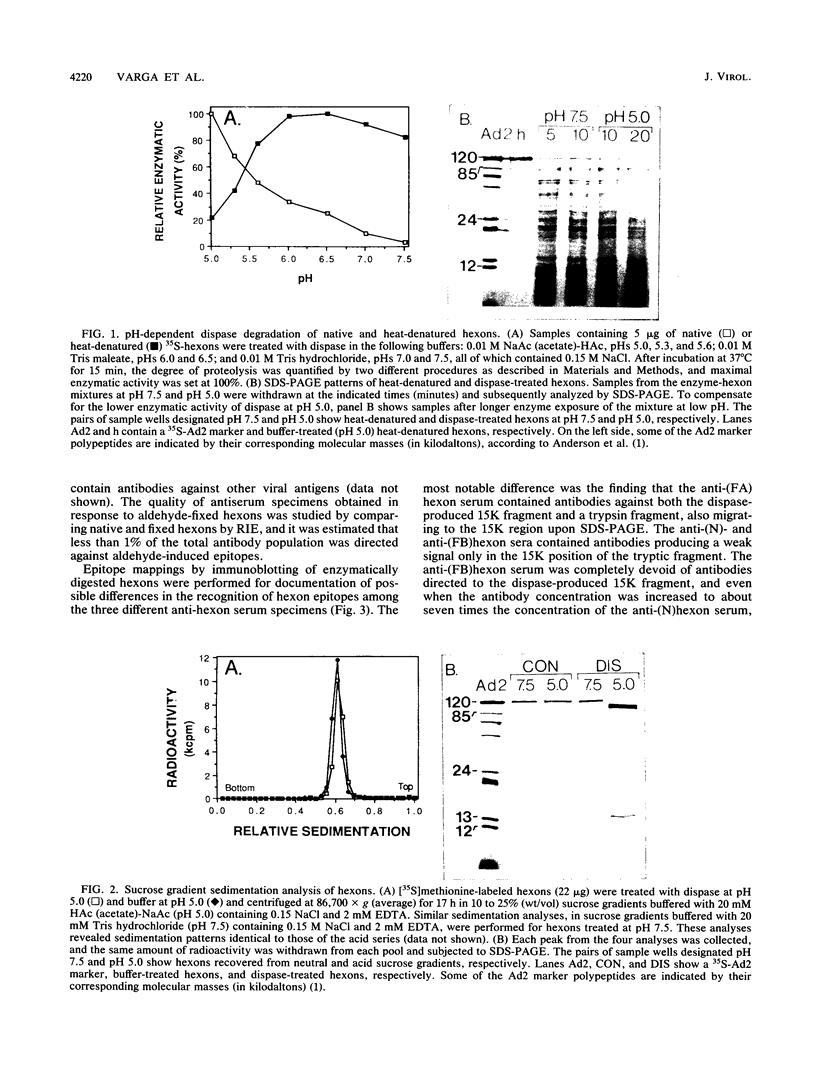
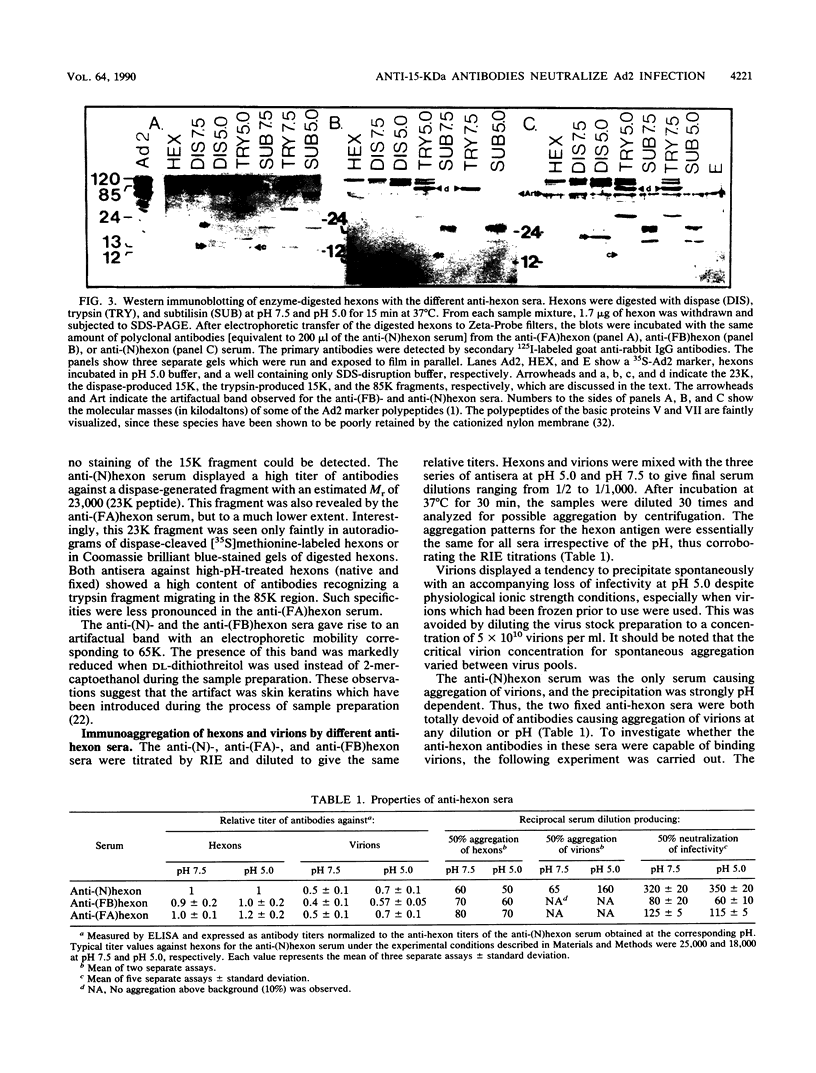
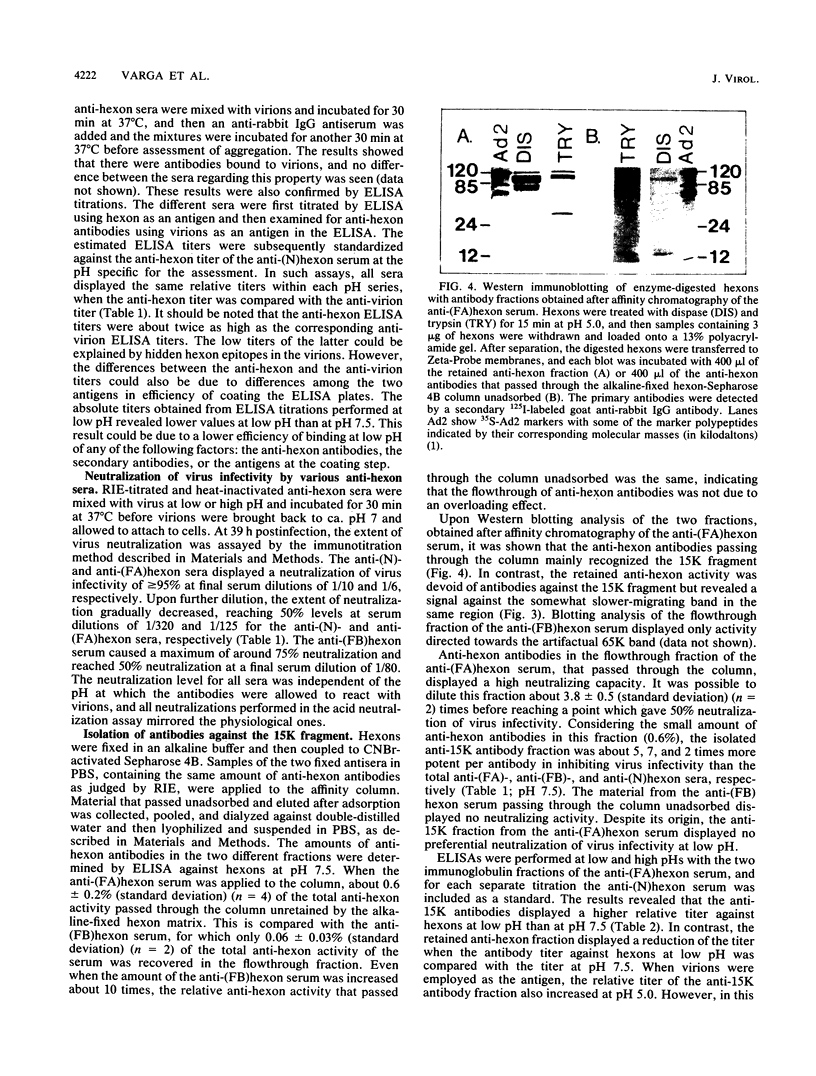
During the entrance of adenovirus type 2 into cells, it has been suggested that the virion undergoes a conformational change. In this investigation, we have further characterized the hypothetical conformational change, which the structural protein hexon undergoes in response to low pH. From pH 5.0 to pH 6.0, the proteolytic enzyme dispase cleaved the hexon into a few distinct fragments with a dominating low-molecular-weight fragment with a molecular weight of 15,000 (15K peptide), whereas between pH 6.5 and pH 8.0, the cleavage of the hexon was negligible. The degradation of the hexon with dispase at low pH was not due to an increased activity or alteration of the active site of dispase at low pH. The 15K fragment was identified as a segment of the N-terminal part of the hexon polypeptide beginning at amino acid residue 5. An immune serum produced in response to acid-treated and glutaraldehyde-fixed hexons contained a small amount of antibodies directed towards the 15K fragment, as judged by Western immunoblotting. An anti-15K antibody fraction was isolated by affinity chromatography by removing antibodies recognizing the hexon in the alkaline configuration. Such antibodies displayed a higher relative titer at pH 5.0 than at pH 7.5 in an enzyme-linked immunosorbent assay. The isolated antibodies showed a specific neutralizing capacity five times higher than that of the corresponding unfractionated polyclonal anti-hexon serum; however, the neutralizing ability was independent of pH. The neutralization of adenovirus type 2 infection by the isolated anti-15K antibodies implies that the N-terminal end of the hexon may play a critical role in the early steps of the virion-cell interaction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman T., Jörnvall H. Electroblotting of individual polypeptides from SDS/polyacrylamide gels for direct sequence analysis. Eur J Biochem. 1987 Nov 16;169(1):9–12. doi: 10.1111/j.1432-1033.1987.tb13573.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal R., Seth P., Willingham M. C., Pastan I. pH-dependent lysis of liposomes by adenovirus. Biochemistry. 1986 Apr 22;25(8):2231–2237. doi: 10.1021/bi00356a057. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Burlingham B. T. Penetration of host cell membranes by adenovirus 2. J Virol. 1973 Aug;12(2):386–396. doi: 10.1128/jvi.12.2.386-396.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970 Mar;40(3):462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- Everitt E., Meador S. A., Levine A. S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977 Jan;21(1):199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Philipson L. Mechanism of the arginine requirement for adenovirus synthesis. I. Synthesis of structural proteins. J Virol. 1971 Nov;8(5):742–753. doi: 10.1128/jvi.8.5.742-753.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald D. J., Padmanabhan R., Pastan I., Willingham M. C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983 Feb;32(2):607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gatti R. A., Ostborn A., Fagraeus A. Selective impairment of cell antigenicity by fixation. J Immunol. 1974 Oct;113(4):1361–1368. [PubMed] [Google Scholar]
- Habeeb A. F. A study of the antigenicity of formaldehyde- and glutaraldehyde-treated bovine serum albumin and ovalbumin-bovine serum albumin conjugate. J Immunol. 1969 Feb;102(2):457–465. [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Akusjärvi G., Aleström P., von Bahr-Lindström H., Pettersson U., Appella E., Fowler A. V., Philipson L. The adenovirus hexon protein. The primary structure of the polypeptide and its correlation with the hexon gene. J Biol Chem. 1981 Jun 25;256(12):6181–6186. [PubMed] [Google Scholar]
- Kjellén L., Pereira H. G. Role of adenovirus antigens in the induction of virus neutralizing antibody. J Gen Virol. 1968 Jan;2(1):177–185. doi: 10.1099/0022-1317-2-1-177. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Mednis B. Structure and development of viruses as observed in the electron microscope. V. Entry and uncoating of adenovirus. J Virol. 1969 Nov;4(5):777–796. doi: 10.1128/jvi.4.5.777-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs D. Protein contaminants of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1983 Dec;135(2):470–474. doi: 10.1016/0003-2697(83)90714-5. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Höglund S., Lonberg-Holm K., Philipson L. Structural proteins of adenoviruses. IV. Sequential degradation of the adenovirus type 2 virion. Virology. 1970 Oct;42(2):341–358. doi: 10.1016/0042-6822(70)90278-3. [DOI] [PubMed] [Google Scholar]
- Roberts M. M., White J. L., Grütter M. G., Burnett R. M. Three-dimensional structure of the adenovirus major coat protein hexon. Science. 1986 May 30;232(4754):1148–1151. doi: 10.1126/science.3704642. [DOI] [PubMed] [Google Scholar]
- Seth P., Willingham M. C., Pastan I. Adenovirus-dependent release of 51Cr from KB cells at an acidic pH. J Biol Chem. 1984 Dec 10;259(23):14350–14353. [PubMed] [Google Scholar]
- Seth P., Willingham M. C., Pastan I. Binding of adenovirus and its external proteins to Triton X-114. Dependence on pH. J Biol Chem. 1985 Nov 25;260(27):14431–14434. [PubMed] [Google Scholar]
- Svensson U. Role of vesicles during adenovirus 2 internalization into HeLa cells. J Virol. 1985 Aug;55(2):442–449. doi: 10.1128/jvi.55.2.442-449.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G. Sensitization and neutralization of adenovirus by specific sera against capsid subunits. J Immunol. 1972 Mar;108(3):622–632. [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Wohlfart C. E., Everitt E. Assessment of specific virus infectivity and virus neutralization by a progeny virus immunotitration method as exemplified in an adenovirus system. J Virol Methods. 1985 Jul;11(3):241–251. doi: 10.1016/0166-0934(85)90113-2. [DOI] [PubMed] [Google Scholar]
- Wohlfart C. E., Svensson U. K., Everitt E. Interaction between HeLa cells and adenovirus type 2 virions neutralized by different antisera. J Virol. 1985 Dec;56(3):896–903. doi: 10.1128/jvi.56.3.896-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfart C., Everitt E. Human adenovirus 2 as immunogen in rabbits yields antisera with high titers of antibodies against the nonstructural 72K DNA-binding protein. Virus Res. 1985 Jul;3(1):77–85. doi: 10.1016/0168-1702(85)90043-7. [DOI] [PubMed] [Google Scholar]
- Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol. 1988 Jul;62(7):2321–2328. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]






