Abstract
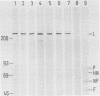
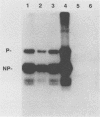
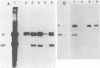
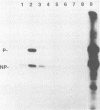
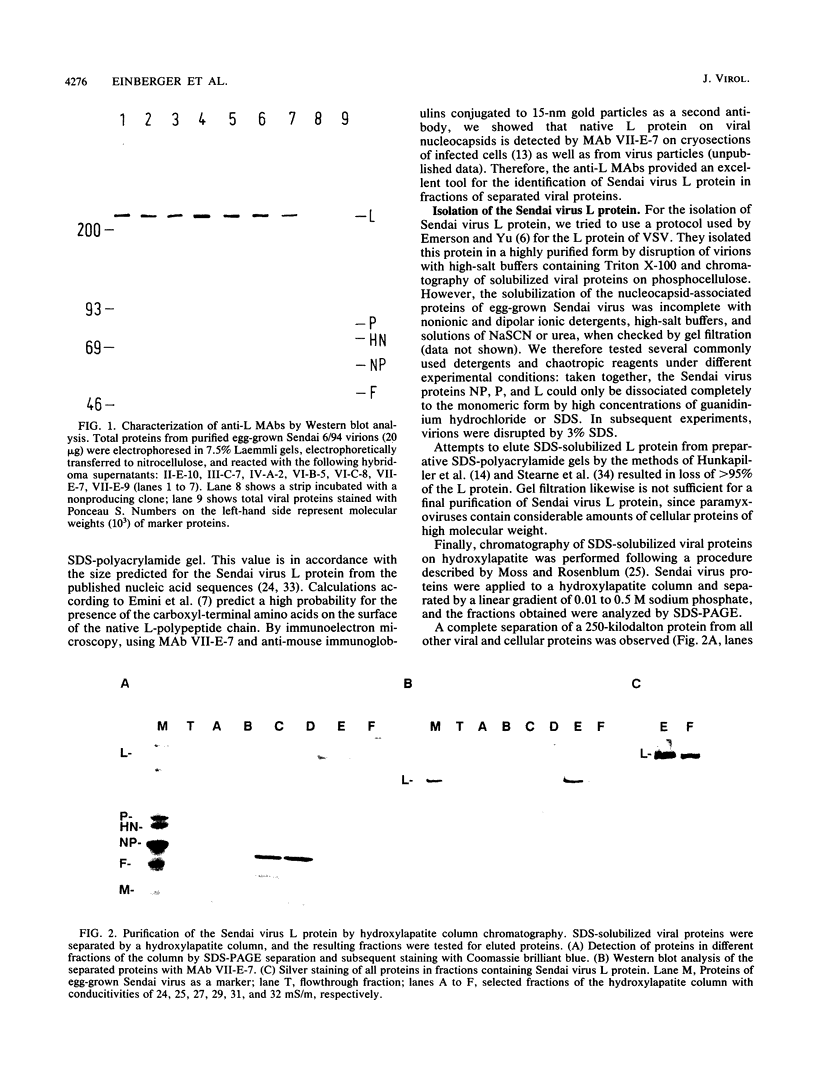
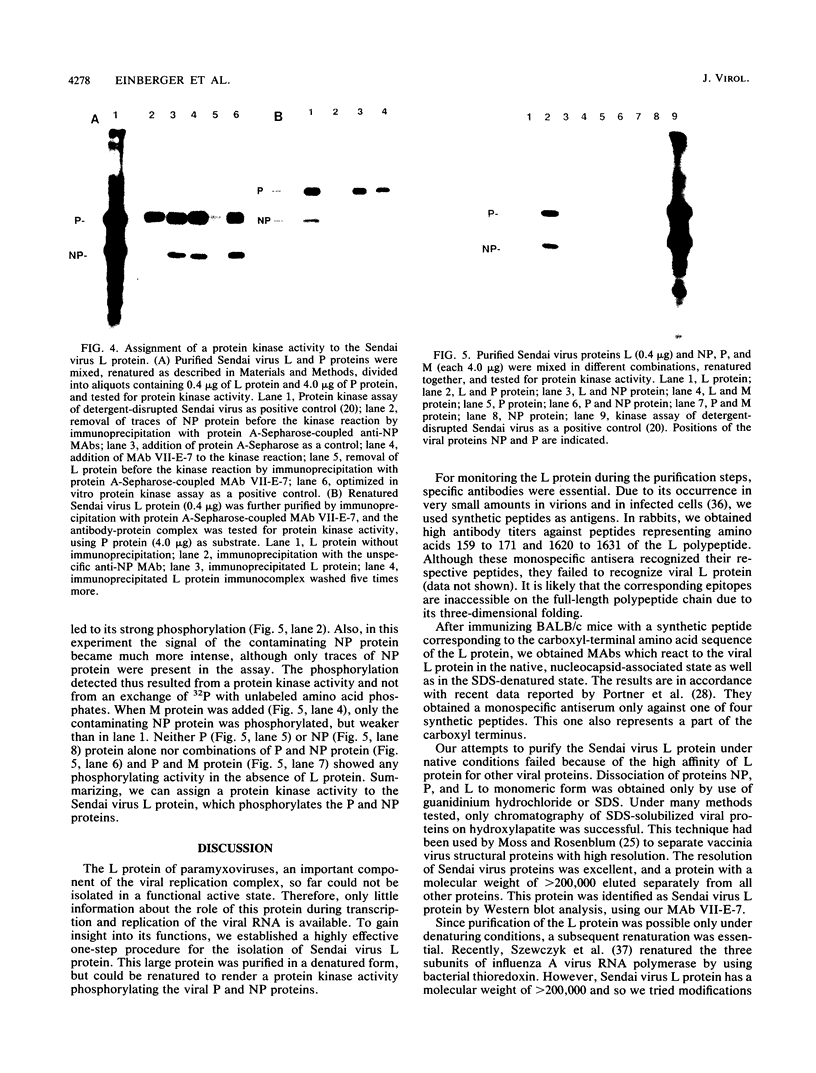
Sodium dodecyl sulfate-solubilized Sendai virus large (L) protein was highly purified by a one-step procedure, using hydroxylapatite column chromatography. Monoclonal antibodies addressed to the carboxyl-terminal amino acid sequence of the L protein were used for monitoring L protein during purification. By removing sodium dodecyl sulfate from purified L protein, a protein kinase activity was successfully renatured. P and NP proteins served as its substrates. After immunoprecipitation with anti-L antibodies, the immunocomplex already showed protein kinase activity. In the presence of P protein, the NP protein was more highly phosphorylated. The results show that Sendai virus L protein possesses a protein kinase activity phosphorylating the other proteins of the viral nucleocapsid in vitro.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckes J. D., Childers L. C., Perrault J. Phosphorylation of vesicular stomatitis virus M protein: evidence for a second virion-associated protein serine kinase activity. Virology. 1989 Mar;169(1):161–171. doi: 10.1016/0042-6822(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Carlsen S. R., Peluso R. W., Moyer S. A. In vitro replication of Sendai virus wild-type and defective interfering particle genome RNAs. J Virol. 1985 May;54(2):493–500. doi: 10.1128/jvi.54.2.493-500.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Hughes J. V., Perlow D. S., Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985 Sep;55(3):836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Yoshida T., Nishikawa K., Naruse H., Nagai Y. Transcriptive complex of Newcastle disease virus. I. Both L and P proteins are required to constitute an active complex. Virology. 1983 Jul 15;128(1):105–117. doi: 10.1016/0042-6822(83)90322-7. [DOI] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W. Topography of phosphate residues in Sendai virus proteins. Virology. 1982 Jul 15;120(1):225–234. doi: 10.1016/0042-6822(82)90020-4. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Lamb R. A. The phosphorylation of sendai virus proteins by a virus particle-associated protein kinase. J Gen Virol. 1975 Mar;26(3):249–263. doi: 10.1099/0022-1317-26-3-249. [DOI] [PubMed] [Google Scholar]
- Massey D. M., Deans N., Lenard J. Phosphorylation of NS protein by vesicular stomatitis virus nucleocapsids: lack of effect during RNA synthesis and separation of kinase from L protein. J Virol. 1990 Jul;64(7):3259–3264. doi: 10.1128/jvi.64.7.3259-3264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meienhofer J., Waki M., Heimer E. P., Lambros T. J., Makofske R. C., Chang C. D. Solid phase synthesis without repetitive acidolysis. Preparation of leucyl-alanyl-glycyl-valine using 9-fluorenylmethyloxycarbonylamino acids. Int J Pept Protein Res. 1979 Jan;13(1):35–42. [PubMed] [Google Scholar]
- Morgan E. M., Rakestraw K. M. Sequence of the Sendai virus L gene: open reading frames upstream of the main coding region suggest that the gene may be polycistronic. Virology. 1986 Oct 15;154(1):31–40. doi: 10.1016/0042-6822(86)90427-7. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Hydroxylapatite chromatography of protein-sodium dodecyl sulfate complexes. A new method for the separation of polypeptide subunits. J Biol Chem. 1972 Aug 25;247(16):5194–5198. [PubMed] [Google Scholar]
- Neubert W. J., Hofschneider P. H., Koprowski H. Search for Sendai 6/94 viral RNA in the antigen-free cell line Cl-C-2 isolated from human multiple sclerosis brain tissue. Infect Immun. 1983 Aug;41(2):675–682. doi: 10.1128/iai.41.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donald P. Measuring the change of population fitness by natural selection. Nature. 1970 Jul 18;227(5255):307–308. doi: 10.1038/227307a0. [DOI] [PubMed] [Google Scholar]
- Portner A., Murti K. G., Morgan E. M., Kingsbury D. W. Antibodies against Sendai virus L protein: distribution of the protein in nucleocapsids revealed by immunoelectron microscopy. Virology. 1988 Mar;163(1):236–239. doi: 10.1016/0042-6822(88)90257-7. [DOI] [PubMed] [Google Scholar]
- Portner A. Synthesis of message and genome RNAs in vitro by Sendai virus-infected cell nucleocapsids. J Gen Virol. 1982 May;60(Pt 1):67–75. doi: 10.1099/0022-1317-60-1-67. [DOI] [PubMed] [Google Scholar]
- Roux L., Kolakofsky D. Protein kinase associated with Sendai virions. J Virol. 1974 Feb;13(2):545–547. doi: 10.1128/jvi.13.2.545-547.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schneider C., Newman R. A., Sutherland D. R., Asser U., Greaves M. F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982 Sep 25;257(18):10766–10769. [PubMed] [Google Scholar]
- Shioda T., Iwasaki K., Shibuta H. Determination of the complete nucleotide sequence of the Sendai virus genome RNA and the predicted amino acid sequences of the F, HN and L proteins. Nucleic Acids Res. 1986 Feb 25;14(4):1545–1563. doi: 10.1093/nar/14.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearne P. A., van Driel I. R., Grego B., Simpson R. J., Goding J. W. The murine plasma cell antigen PC-1: purification and partial amino acid sequence. J Immunol. 1985 Jan;134(1):443–448. [PubMed] [Google Scholar]
- Stewart W. E., 2nd, De Somer P., De Clercq E. Renaturation of inactivated interferons by "defensive reversible denaturation". Prep Biochem. 1974;4(5):383–393. doi: 10.1080/00327487408061543. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Strauss J. H. Replication strategies of the single stranded RNA viruses of eukaryotes. Curr Top Microbiol Immunol. 1983;105:1–98. doi: 10.1007/978-3-642-69159-1_1. [DOI] [PubMed] [Google Scholar]
- Szewczyk B., Laver W. G., Summers D. F. Purification, thioredoxin renaturation, and reconstituted activity of the three subunits of the influenza A virus RNA polymerase. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7907–7911. doi: 10.1073/pnas.85.21.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez A., De B. P., Banerjee A. K. In vitro phosphorylation of NS protein by the L protein of vesicular stomatitis virus. J Gen Virol. 1985 May;66(Pt 5):1025–1036. doi: 10.1099/0022-1317-66-5-1025. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]