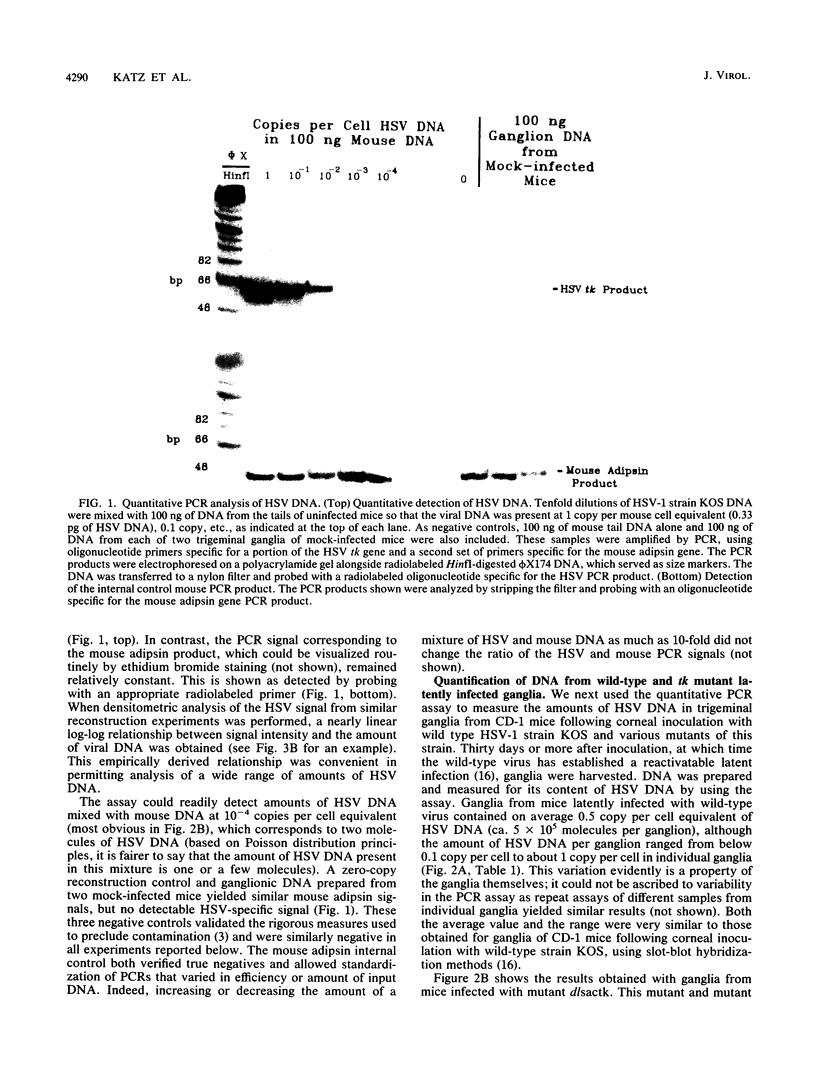
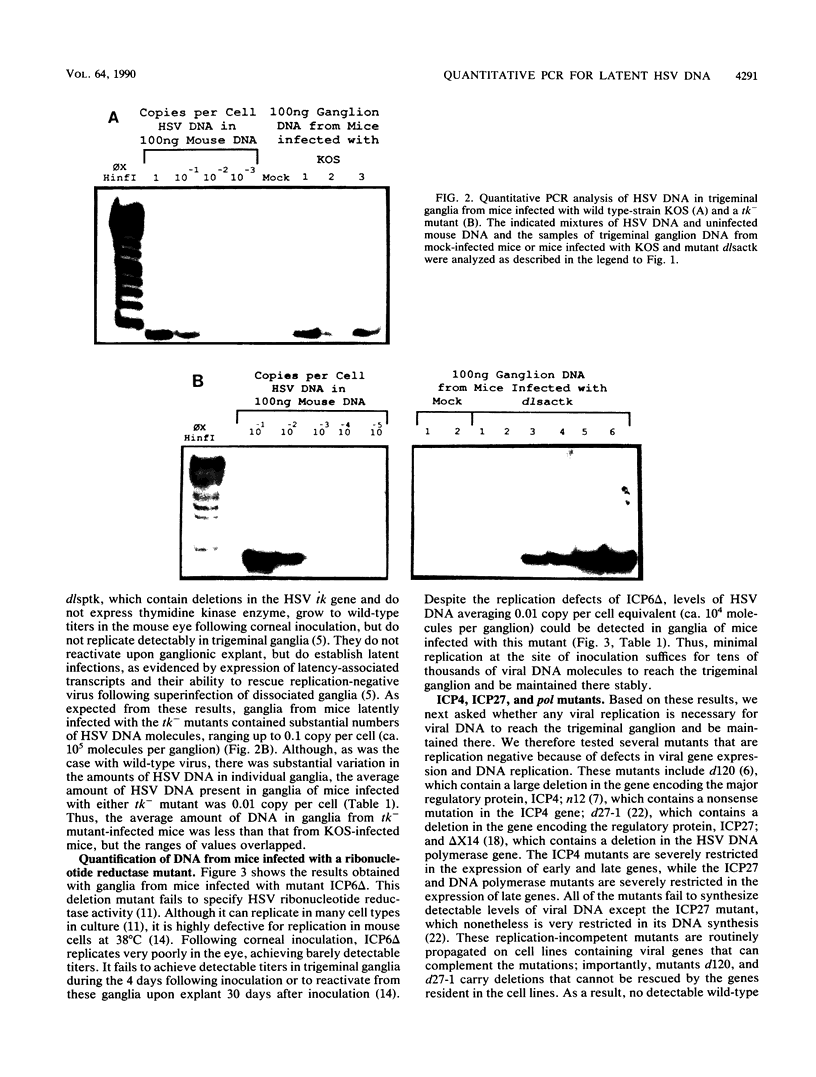
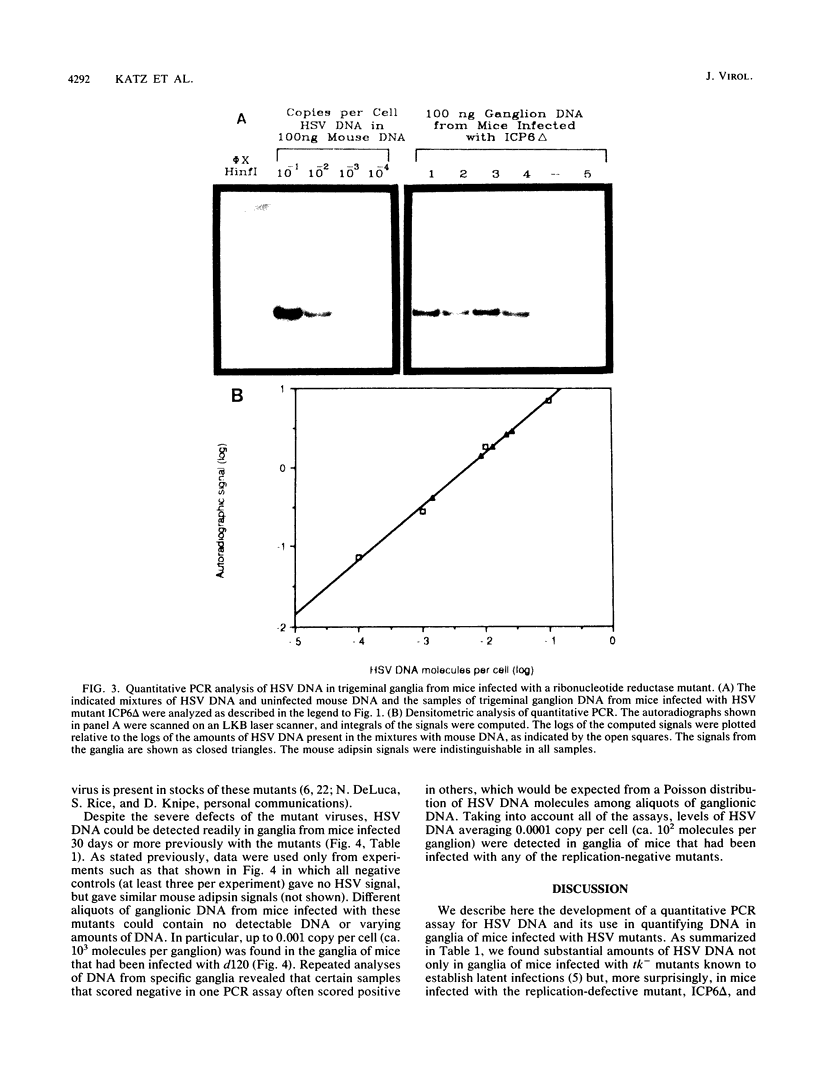
Abstract
To study the roles of viral genes in the establishment and maintenance of herpes simplex virus (HSV) latency, we have developed a polymerase chain reaction assay that is both quantitative and sensitive. Using this assay, we analyzed the levels of viral DNA in trigeminal ganglia of mice inoculated corneally with HSV mutants that are defective for virus replication at one or more sites in mice and for reactivation upon ganglionic explant. Ganglia from mice infected with thymidine kinase-negative mutants, which replicate at the site of inoculation and establish latency but do not replicate acutely in ganglia or reactivate upon explant, contained a range of levels of HSV DNA that overlapped with the range found in ganglia latently infected with wild-type virus. On average, these mutant-infected ganglia contained one copy of HSV DNA per 100 cell equivalents (ca. 10(4) molecules), which was 50-fold less than the average for wild-type virus. Ganglia from mice infected with a ribonucleotide reductase deletion mutant, which is defective for acute replication and reactivation upon ganglionic explant, also contained on average one copy of HSV DNA per 100 cell equivalents. We also detected substantial numbers of HSV DNA molecules (up to ca. 10(3] in ganglia of mice infected with an ICP4 deletion mutant and other replication-negative mutants that are severely impaired for viral DNA replication and gene expression. These results raise the possibility that such mutants can establish latency, which could have important implications for mechanisms of latency and for vaccine and antiviral drug development.
Full text
PDF

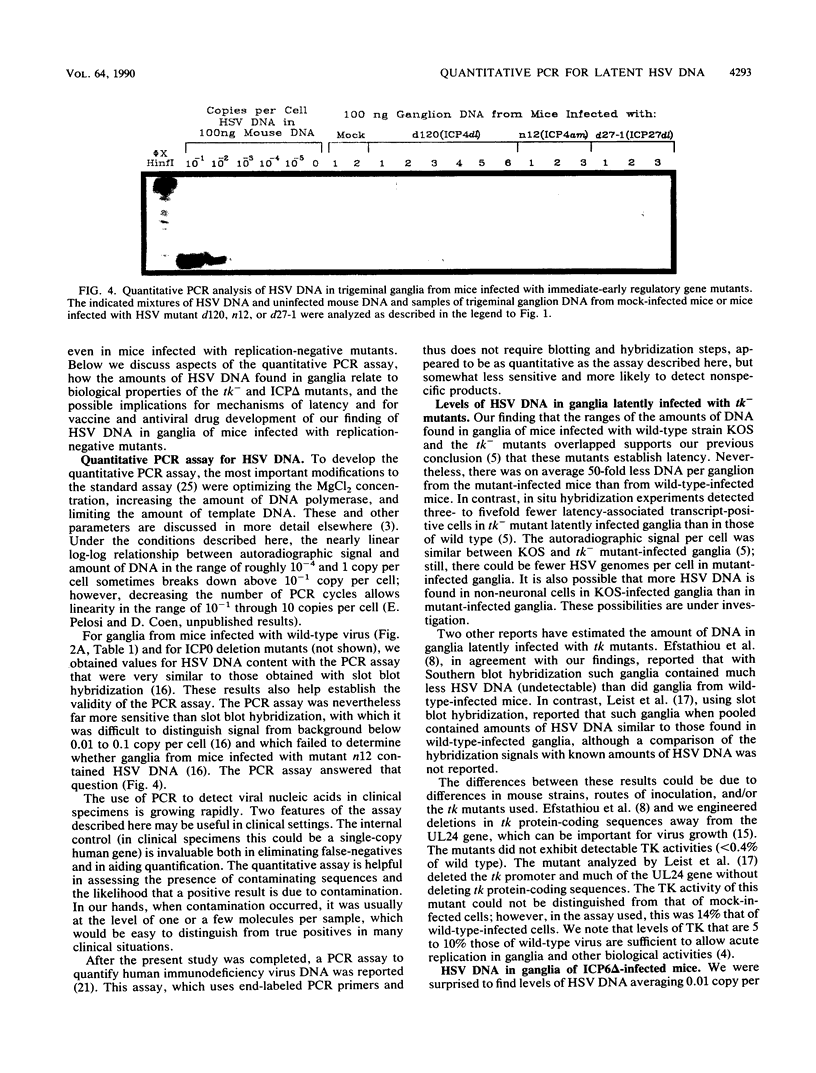


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coen D. M., Irmiere A. F., Jacobson J. G., Kerns K. M. Low levels of herpes simplex virus thymidine- thymidylate kinase are not limiting for sensitivity to certain antiviral drugs or for latency in a mouse model. Virology. 1989 Feb;168(2):221–231. doi: 10.1016/0042-6822(89)90261-4. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Kosz-Vnenchak M., Jacobson J. G., Leib D. A., Bogard C. L., Schaffer P. A., Tyler K. L., Knipe D. M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., McCarthy A. M., Schaffer P. A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985 Nov;56(2):558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou S., Kemp S., Darby G., Minson A. C. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J Gen Virol. 1989 Apr;70(Pt 4):869–879. doi: 10.1099/0022-1317-70-4-869. [DOI] [PubMed] [Google Scholar]
- Efstathiou S., Minson A. C., Field H. J., Anderson J. R., Wildy P. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in humans. J Virol. 1986 Feb;57(2):446–455. doi: 10.1128/jvi.57.2.446-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Weller S. K. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988 Sep;166(1):41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Weller S. K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988 Jan;62(1):196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmiere A. F., Manos M. M., Jacobson J. G., Gibbs J. S., Coen D. M. Effect of an amber mutation in the herpes simplex virus thymidine kinase gene on polypeptide synthesis and stability. Virology. 1989 Feb;168(2):210–220. doi: 10.1016/0042-6822(89)90260-2. [DOI] [PubMed] [Google Scholar]
- Jacobson J. G., Leib D. A., Goldstein D. J., Bogard C. L., Schaffer P. A., Weller S. K., Coen D. M. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology. 1989 Nov;173(1):276–283. doi: 10.1016/0042-6822(89)90244-4. [DOI] [PubMed] [Google Scholar]
- Jacobson J. G., Martin S. L., Coen D. M. A conserved open reading frame that overlaps the herpes simplex virus thymidine kinase gene is important for viral growth in cell culture. J Virol. 1989 Apr;63(4):1839–1843. doi: 10.1128/jvi.63.4.1839-1843.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D. A., Coen D. M., Bogard C. L., Hicks K. A., Yager D. R., Knipe D. M., Tyler K. L., Schaffer P. A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989 Feb;63(2):759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist T. P., Sandri-Goldin R. M., Stevens J. G. Latent infections in spinal ganglia with thymidine kinase-deficient herpes simplex virus. J Virol. 1989 Nov;63(11):4976–4978. doi: 10.1128/jvi.63.11.4976-4978.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcy A. I., Yager D. R., Coen D. M. Isolation and characterization of herpes simplex virus mutants containing engineered mutations at the DNA polymerase locus. J Virol. 1990 May;64(5):2208–2216. doi: 10.1128/jvi.64.5.2208-2216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S., Koyanagi Y., Miles S., Wiley C., Vinters H. V., Chen I. S. High levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patients. Nature. 1990 Jan 4;343(6253):85–89. doi: 10.1038/343085a0. [DOI] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990 Apr;64(4):1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983 Apr 7;302(5908):523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Deshmane S. L., Ace C. I., Preston C. M., Fraser N. W. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J Virol. 1990 Apr;64(4):1630–1638. doi: 10.1128/jvi.64.4.1630-1638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989 Sep;53(3):318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Hay K. A., Edris W. A. Latency-associated transcript but not reactivatable virus is present in sensory ganglion neurons after inoculation of thymidine kinase-negative mutants of herpes simplex virus type 1. J Virol. 1989 Jun;63(6):2861–2865. doi: 10.1128/jvi.63.6.2861-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Aschman D. P., Sacks W. R., Coen D. M., Schaffer P. A. Genetic analysis of temperature-sensitive mutants of HSV-1: the combined use of complementation and physical mapping for cistron assignment. Virology. 1983 Oct 30;130(2):290–305. doi: 10.1016/0042-6822(83)90084-3. [DOI] [PubMed] [Google Scholar]