Abstract
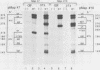
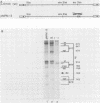
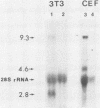
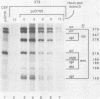
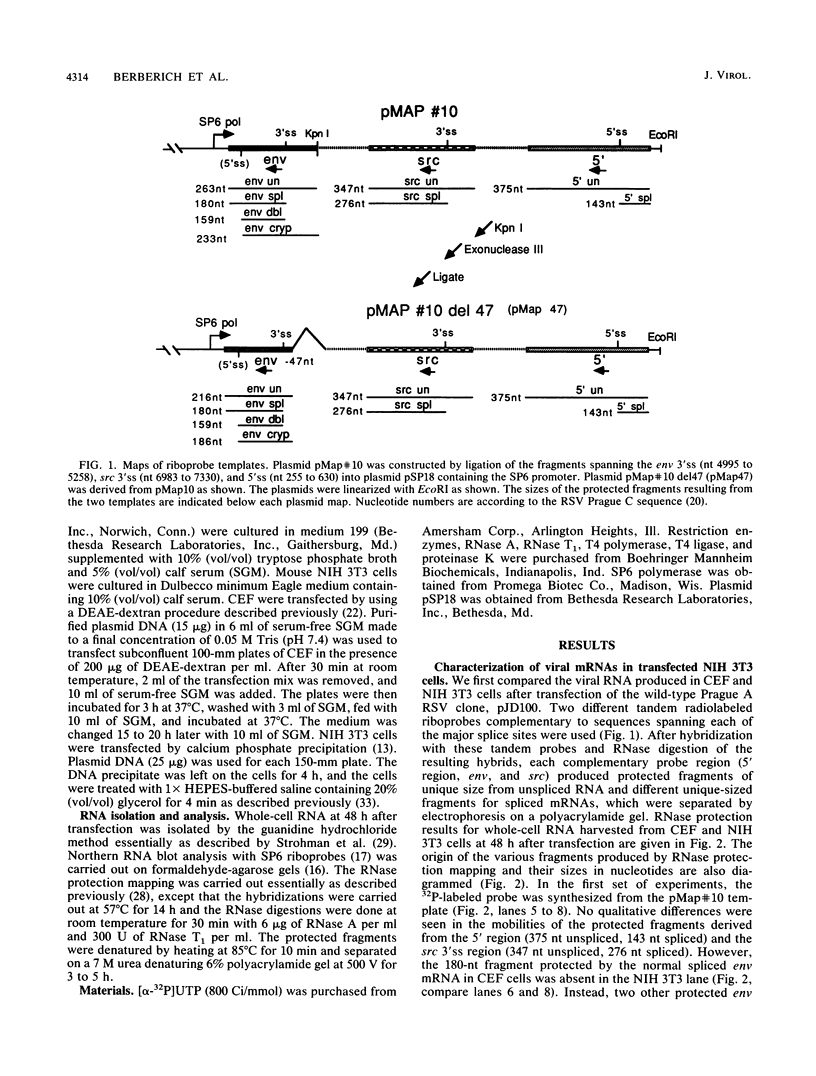
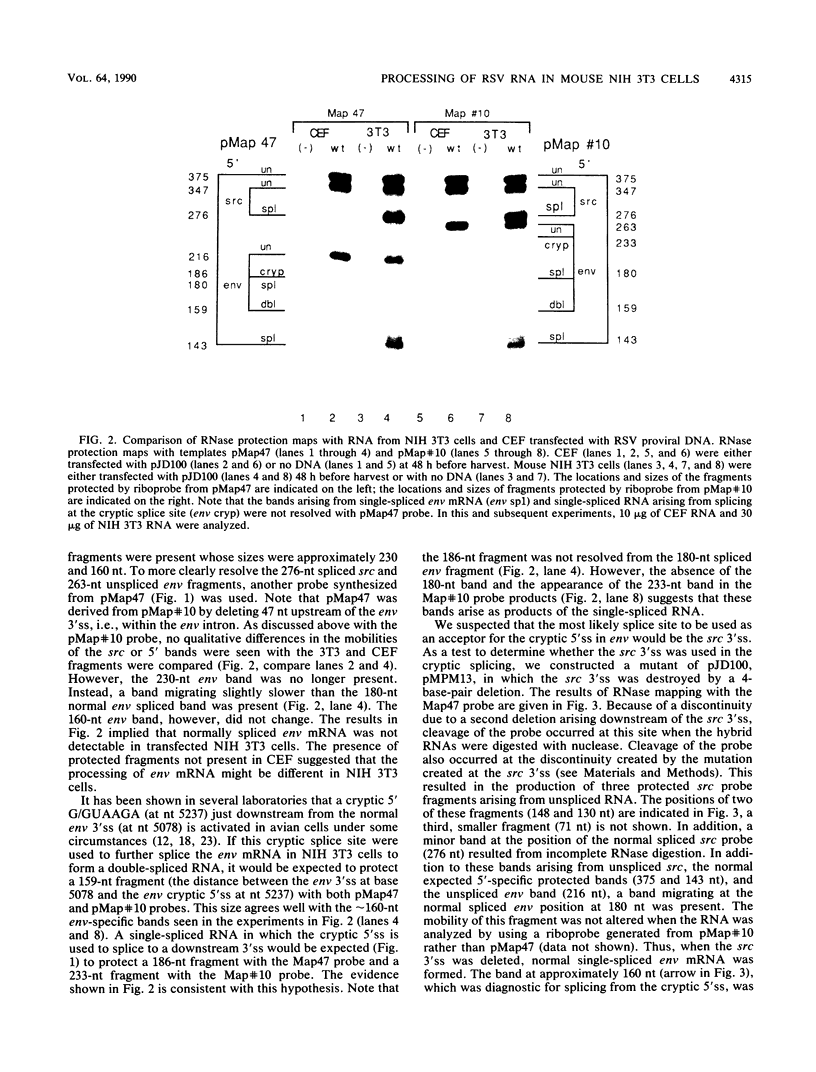
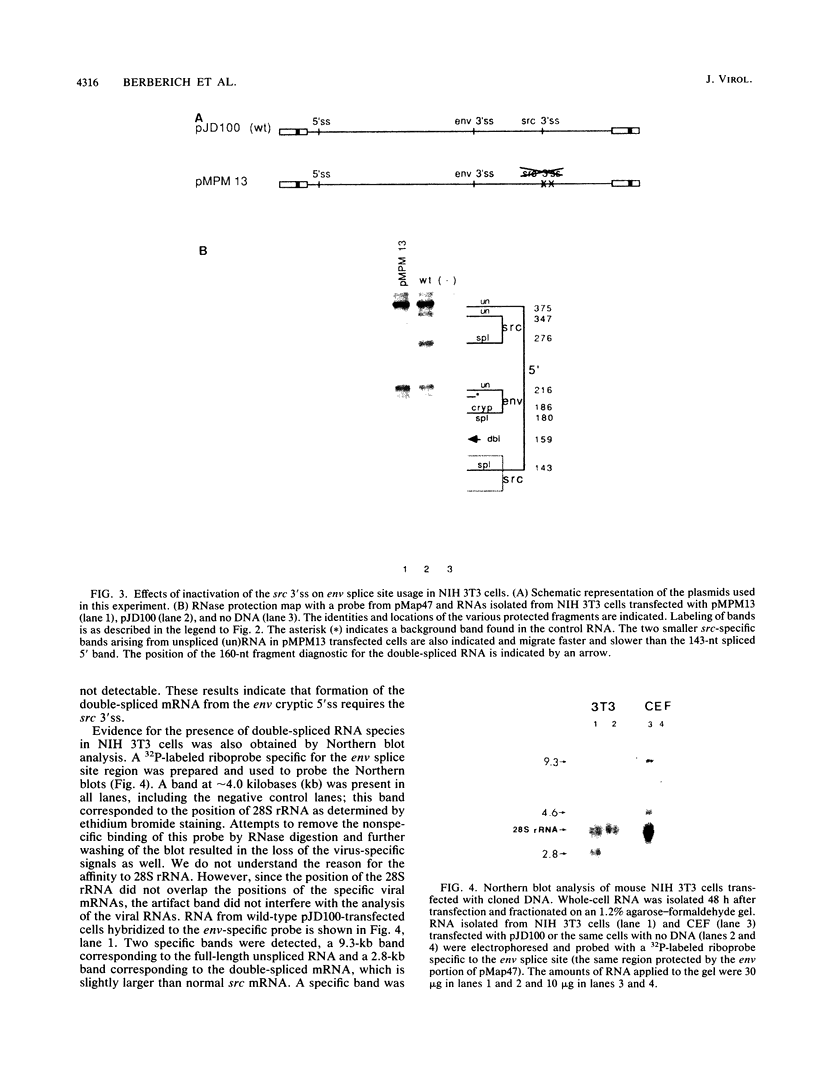
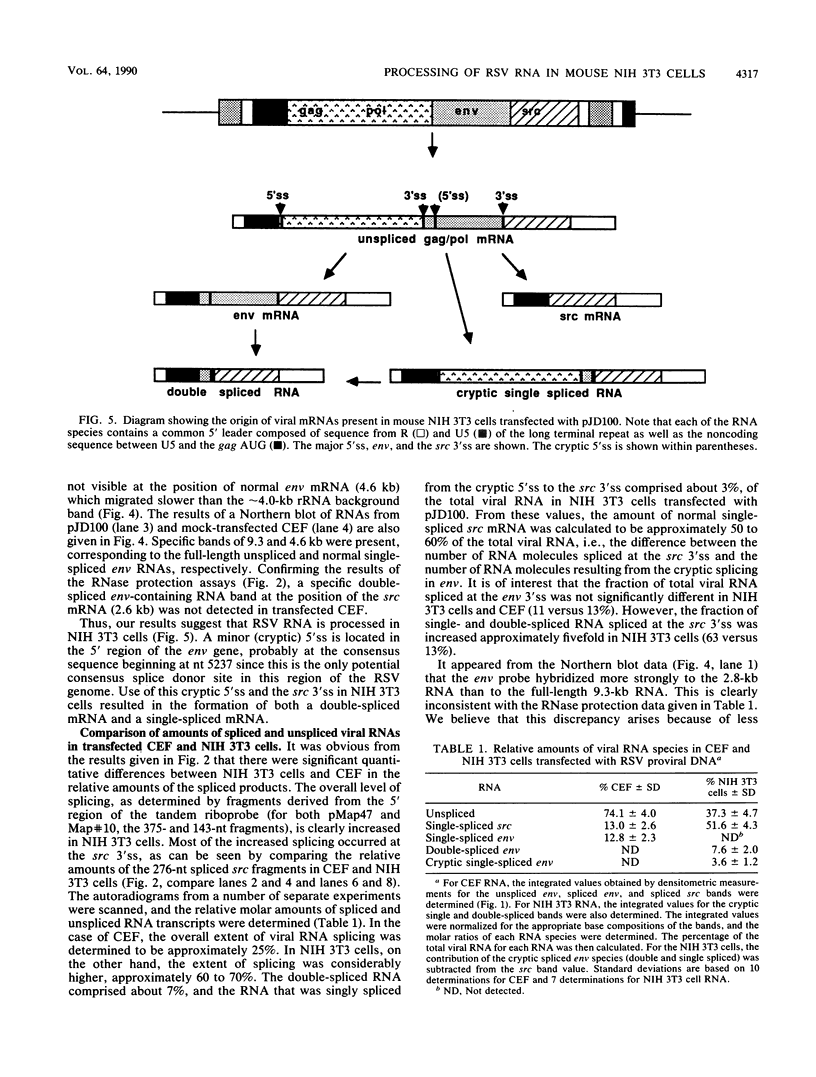
Rous sarcoma virus, an avian retrovirus, transforms but does not replicate in mammalian cells. To determine to what extent differences in RNA splicing might contribute to this lack of productive infection, cloned proviral DNA derived from the Prague A strain of Rous sarcoma virus was transfected into mouse NIH 3T3 cells, and the viral RNA was compared by RNase protection with viral RNA from transfected chicken embryo fibroblasts by using a tandem antisense riboprobe spanning the three major splice sites. The levels of viral RNA in NIH 3T3 cells compared with those in chicken embryo fibroblasts were lower, but the RNA was spliced at increased efficiency. The difference in the ratio of unspliced to spliced RNA levels was not due to the increased lability of unspliced RNA in NIH 3T3 cells. Although chicken embryo fibroblasts contained equal levels of src and env mRNAs, spliced viral mRNAs in NIH 3T3 cells were almost exclusively src. In NIH 3T3 cells the env mRNA was further processed by using a cryptic 5' splice site located within the env coding sequences and the normal src 3' splice site to form a double-spliced mRNA. This mRNA was identical to the src mRNA, except that a 159-nucleotide sequence from the 5' end of the env gene was inserted at the src splice junction. Smaller amounts of single-spliced RNA were also present in which only the region between the cryptic 5' and src 3' splice sites was spliced out. The aberrant processing of the viral env mRNA in NIH 3T3 cells may in part explain the nonpermissiveness of these cells to productive Rous sarcoma virus infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altaner C., Temin H. M. Carcinogenesis by RNA sarcoma viruses. XII. A quantitative study of infection of rat cells in vitro by avian sarcoma viruses. Virology. 1970 Jan;40(1):118–134. doi: 10.1016/0042-6822(70)90384-3. [DOI] [PubMed] [Google Scholar]
- Arrigo S., Beemon K. Regulation of Rous sarcoma virus RNA splicing and stability. Mol Cell Biol. 1988 Nov;8(11):4858–4867. doi: 10.1128/mcb.8.11.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. J., Stoltzfus C. M. Cloning and nucleotide sequences of cDNAs spanning the splice junctions of Rous sarcoma virus mRNAs. J Virol. 1985 Mar;53(3):969–972. doi: 10.1128/jvi.53.3.969-972.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Rescue of rous sarcoma virus from rous sarcoma virus-transformed mammalian cells. J Virol. 1972 Jul;10(1):153–156. doi: 10.1128/jvi.10.1.153-156.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Hybridization of Rous sarcoma virus deoxyribonucleic acid polymerase product and ribonucleic acids from chicken and rat cells infected with Rous sarcoma virus. J Virol. 1972 May;9(5):766–775. doi: 10.1128/jvi.9.5.766-775.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Copeland N. G., Zelenetz A. D., Krontiris T. Transformation of NIH-3T3 mouse cells by avian retroviral DNAs. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1169–1176. doi: 10.1101/sqb.1980.044.01.126. [DOI] [PubMed] [Google Scholar]
- Deng C. T., Boettiger D., Macpherson I., Varmus H. E. The persistence and expression of virus-specific DNA in revertants of Rous sarcoma virus-transformed BHK-21 cells. Virology. 1974 Dec;62(2):512–521. doi: 10.1016/0042-6822(74)90411-5. [DOI] [PubMed] [Google Scholar]
- Deng C. T., Stehelin D., Bishop J. M., Varmus H. E. Characteristics of virus-specific RNA in avian sarcoma virus-transformed BHK-21 cells and revertants. Virology. 1977 Jan;76(1):313–330. doi: 10.1016/0042-6822(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Duff R. G., Vogt P. K. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Varmus H. E., Bishop J. M. Virus-specific messenger RNAs in permissive cells infected by avian sarcoma virus. J Biol Chem. 1979 Aug 25;254(16):8015–8022. [PubMed] [Google Scholar]
- Machala O., Donner L., Svoboda J. A full expression of the genome of Rous sarcoma virus in heterokaryons formed after fusion of virogenic mammalian cells and chicken fibroblasts. J Gen Virol. 1970 Sep;8(3):219–229. doi: 10.1099/0022-1317-8-3-219. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelley R. J., Moscovici C., Hughes S., Kung H. J. Proviral-activated c-erbB is leukemogenic but not sarcomagenic: characterization of a replication-competent retrovirus containing the activated c-erbB. J Virol. 1988 May;62(5):1840–1844. doi: 10.1128/jvi.62.5.1840-1844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintrell N., Hughes S. H., Varmus H. E., Bishop J. M. Structure of viral DNA and RNA in mammalian cells infected with avian sarcoma virus. J Mol Biol. 1980 Nov 15;143(4):363–393. doi: 10.1016/0022-2836(80)90218-1. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Simkovic D. Characteristics of tumors induced in mammals, especially rodents, by viruses of the avian leukosis sarcoma group. Adv Virus Res. 1972;17:95–127. [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7575–7578. doi: 10.1073/pnas.78.12.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong M. M., Iijima S., Wang L. H. Transduction of c-src coding and intron sequences by a transformation-defective deletion mutant of Rous sarcoma virus. J Virol. 1986 Sep;59(3):556–563. doi: 10.1128/jvi.59.3.556-563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer K. S., Boettiger D. Complementation rescue of Rous sarcoma virus from transformed mammalian cells by polyethylene glycol-mediated cell fusion. J Virol. 1977 Jul;23(1):133–141. doi: 10.1128/jvi.23.1.133-141.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Dimock K., Horikami S., Ficht T. A. Stabilities of avian sarcoma virus RNAs: comparison of subgenomic and genomic species with cellular mRNAs. J Gen Virol. 1983 Oct;64(Pt 10):2191–2202. doi: 10.1099/0022-1317-64-10-2191. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Fogarty S. J. Multiple regions in the Rous sarcoma virus src gene intron act in cis to affect the accumulation of unspliced RNA. J Virol. 1989 Apr;63(4):1669–1676. doi: 10.1128/jvi.63.4.1669-1676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M. Synthesis and processing of avian sarcoma retrovirus RNA. Adv Virus Res. 1988;35:1–38. doi: 10.1016/s0065-3527(08)60707-1. [DOI] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Dourmashkin R. Rescue of Rous sarcoma virus from virogenic mammalian cells associated with chicken cells and treated with Sendai virus. J Gen Virol. 1969 Jun;4(4):523–529. doi: 10.1099/0022-1317-4-4-523. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Machala O., Donner L., Sovovã Comparative study of RSV rescue from RSV-transformed mammalian cells. Int J Cancer. 1971 Nov 15;8(3):391–400. doi: 10.1002/ijc.2910080306. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek L. P., Oppermann H. Spontaneous conversion of nontransformed avian sarcoma virus-infected rat cells to the transformed phenotype. J Virol. 1980 Aug;35(2):466–478. doi: 10.1128/jvi.35.2.466-478.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Vogt P. K. Appearance of virus-specific DNA in mammalian cells following transformation by Rous sarcoma virus. J Mol Biol. 1973 Mar 15;74(4):613–626. doi: 10.1016/0022-2836(73)90052-1. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Bruckenstein D. A., Bell A. P. Avian sarcoma virus gag precursor polypeptide is not processed in mammalian cells. J Virol. 1982 Nov;44(2):725–730. doi: 10.1128/jvi.44.2.725-730.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Achacoso J. A. Creation and expression of myristylated forms of Rous sarcoma virus gag protein in mammalian cells. J Virol. 1989 Oct;63(10):4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Helm K., Kempeni J., Wille W., Willecke K. Rous sarcoma virus precursor protein pr 76 is processed in avian sarcoma virus-transformed mammalian cells after fusion-injection of viral protein p 15. Virology. 1980 Oct 30;106(2):310–316. doi: 10.1016/0042-6822(80)90254-8. [DOI] [PubMed] [Google Scholar]