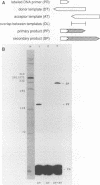
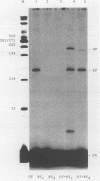
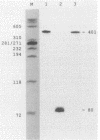
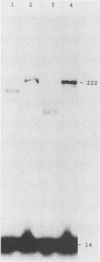
Abstract
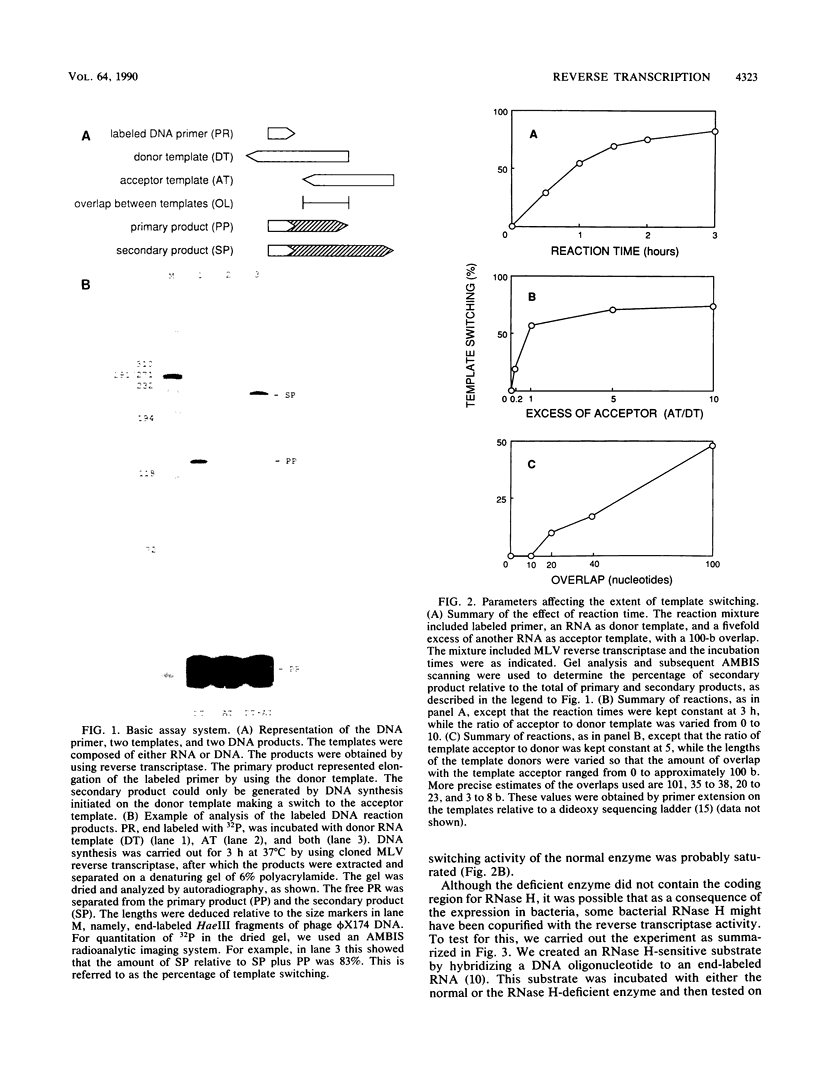
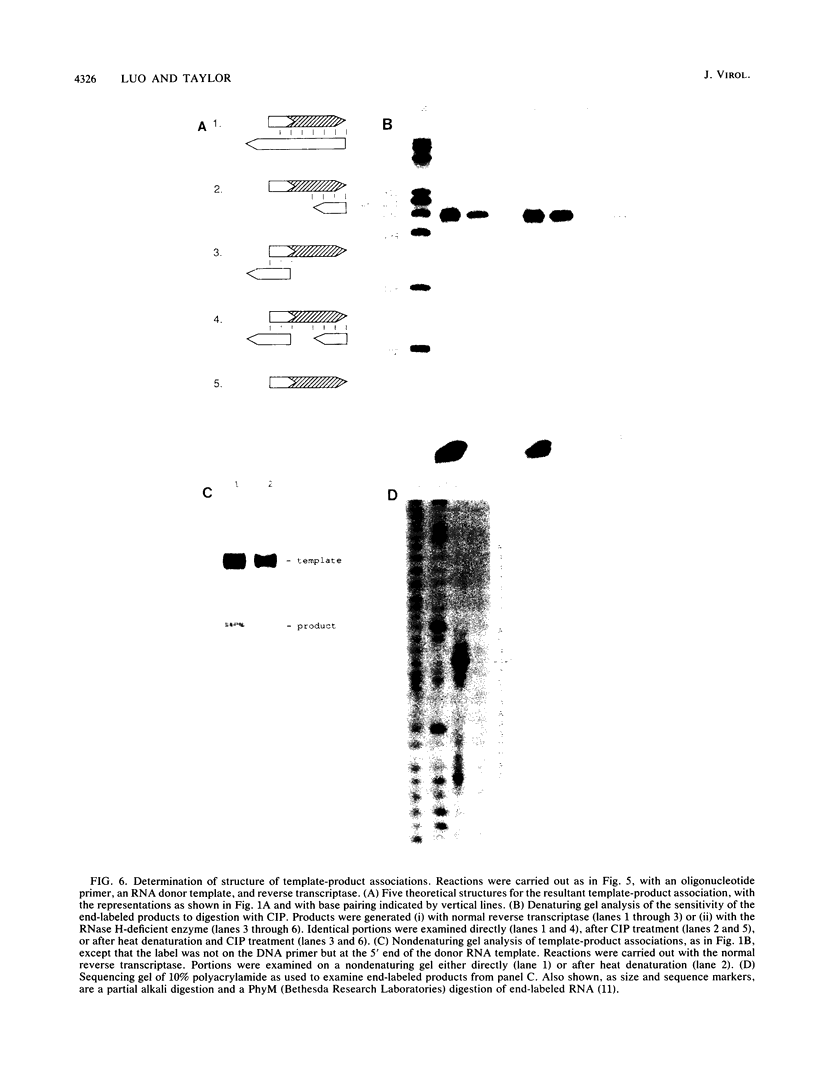
The ability of reverse transcriptase to make template switches during DNA synthesis is implicit in models of retrovirus genome replication, as well as in recombination and oncogene transduction. In order to understand such switching, we used in vitro reactions with purified nucleic acids and enzymes. The assay system involved the use of an end-labeled DNA primer so as to allow the quantitation of elongation on a donor template relative to the amount of elongation achieved by template switching (by means of sequence homology) when an acceptor template RNA was added. We examined several variables that affected the efficiency of the reaction: (i) the reaction time, (ii) the relative amounts of acceptor and donor template, (iii) the extent of sequence overlap between the donor and acceptor templates, and (iv) the presence or absence of RNase H activity associated with the reverse transcriptase. The basic reaction, with RNA templates and normal reverse transcriptase, yielded as much as 83% template switching. In the absence of RNase H, switching still occurred but the efficiency was lowered. Also, when the donor template was changed from RNA to DNA, there was still switching; not surprisingly, this was largely unaffected by the presence or absence of RNase H. Finally, we examined the action of the RNase H on RNA templates after primary transcription but prior to template switching. We found that in most cases, both ends of the original RNA template were able to maintain an association with the DNA product. This result was consistent with the work of others who have shown that RNase H acts as an endonuclease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Leis J. P., Smith M. S., Faras A. J. Unwinding-like activity associated with avian retrovirus RNA-directed DNA polymerase. J Virol. 1978 May;26(2):498–509. doi: 10.1128/jvi.26.2.498-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard G. F., D'Alessio J. M., Kotewicz M. L., Noon M. C. Influence on stability in Escherichia coli of the carboxy-terminal structure of cloned Moloney murine leukemia virus reverse transcriptase. DNA. 1986 Aug;5(4):271–279. doi: 10.1089/dna.1986.5.271. [DOI] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Retroviral recombination during reverse transcription. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2052–2056. doi: 10.1073/pnas.87.6.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1556–1560. doi: 10.1073/pnas.87.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Boone L. R., Skalka A. M. Retroviral DNA H structures: displacement-assimilation model of recombination. Cell. 1982 Aug;30(1):53–62. doi: 10.1016/0092-8674(82)90011-3. [DOI] [PubMed] [Google Scholar]
- Kotewicz M. L., Sampson C. M., D'Alessio J. M., Gerard G. F. Isolation of cloned Moloney murine leukemia virus reverse transcriptase lacking ribonuclease H activity. Nucleic Acids Res. 1988 Jan 11;16(1):265–277. doi: 10.1093/nar/16.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M. S., Berger S. L. Ribonuclease H activities associated with viral reverse transcriptases are endonucleases. Proc Natl Acad Sci U S A. 1989 May;86(10):3539–3543. doi: 10.1073/pnas.86.10.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien J. M., Aldrich C. E., Mason W. S. Evidence that a capped oligoribonucleotide is the primer for duck hepatitis B virus plus-strand DNA synthesis. J Virol. 1986 Jan;57(1):229–236. doi: 10.1128/jvi.57.1.229-236.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G. X., Sharmeen L., Taylor J. Specificities involved in the initiation of retroviral plus-strand DNA. J Virol. 1990 Feb;64(2):592–597. doi: 10.1128/jvi.64.2.592-597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Taylor J. M., Hull R. Retroid virus genome replication. Adv Virus Res. 1987;32:35–96. doi: 10.1016/s0065-3527(08)60474-1. [DOI] [PubMed] [Google Scholar]
- Oyama F., Kikuchi R., Crouch R. J., Uchida T. Intrinsic properties of reverse transcriptase in reverse transcription. Associated RNase H is essentially regarded as an endonuclease. J Biol Chem. 1989 Nov 5;264(31):18808–18817. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science. 1986 Apr 25;232(4749):477–484. doi: 10.1126/science.3961490. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]