Abstract
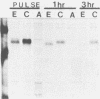
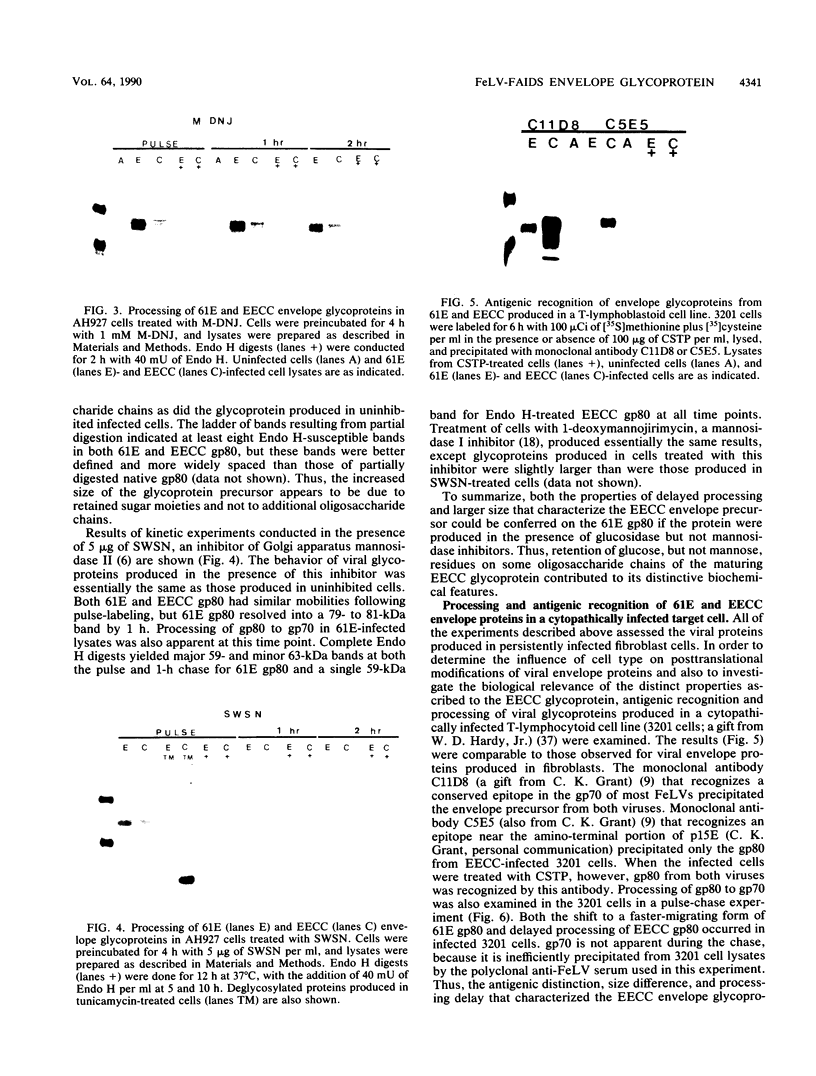
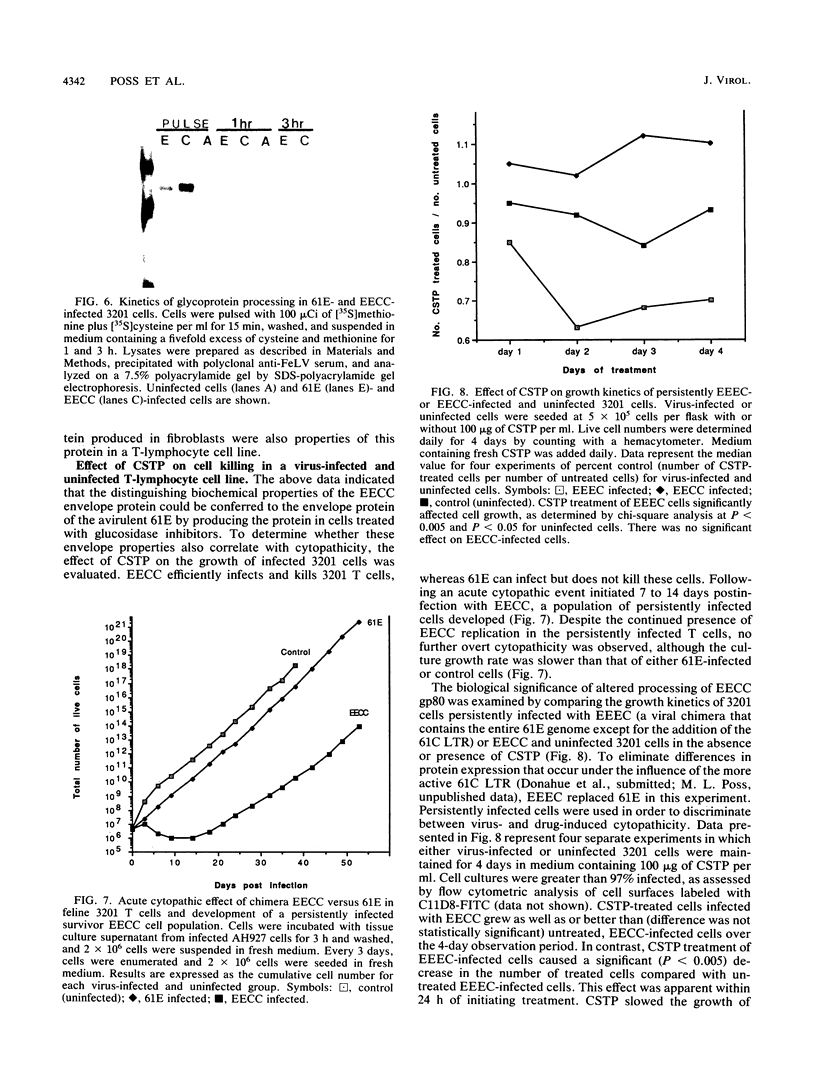
FeLV-FAIDS, an immunodeficiency-inducing isolate of feline leukemia virus, is composed of a pathogenic but replication-defective genome (molecular clone 61C) and a replication-competent but non-immunodeficiency-inducing variant genome (molecular clone 61E). The chimeric virus EECC, composed of the 5' gag-pol of 61E fused to the env-3' LTR of 61C, also induces immunodeficiency. The 61C (or EECC) gp80 can be distinguished from that of 61E on the basis of antigenic recognition, size, and rate of posttranslational processing. We found that the nascent precursor polypeptides of the two viruses were the same size; however, the 61E gp80 rapidly shifted to a smaller size and was subsequently cleaved to gp70, whereas EECC gp80 maintained its nascent size and was cleaved to gp70 only after a prolonged time. Endo-beta-N-acetyl glucosaminidase H and N-glycanase digestions of newly formed glycoproteins resulted in a similar banding pattern for both viruses, indicating that both contained the same number of oligosaccharide side chains and that all of these were high mannose sugars. The metabolic inhibitors of glycosylation, castanospermine or N-methyldeoxynojirimycin, prevented both the rapid trimming of 61E gp80 and its cleavage to gp70. Treatment with mannosidase inhibitors, however, did not affect 61E gp80 processing or size, suggesting that retention of glucose residues on EECC was responsible for these distinguishing properties of the glycoprotein. The pathological consequence of aberrant viral glycoprotein processing was evaluated in feline 3201 T lymphocytes, which are infectable by both 61E and EECC but are killed only by EECC. As in fibroblasts, the EECC glycoprotein produced in lymphocytes was larger, antigenically distinct, and processed more slowly than was the glycoprotein of 61E. Castanospermine treatment of 61E-infected 3201 T cells, however, not only abrogated the antigenic differences between the 61E and EECC glycoproteins but also resulted in a cytopathic effect. Our results suggest that (i) intracellular accumulation of EECC envelope glycoprotein may occur consequent to retention of glucose residues on carbohydrate side chains and (ii) a strong correlation exists between delayed glycoprotein processing and cytopathicity in FeLV-FAIDS-infected T lymphocytes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. R., Seely J. E., Klemcke H. G., Hughes J. P. Receptor binding and Nb2 cell mitogenic activities of glycosylated vs. unglycosylated porcine prolactin. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1187–1193. doi: 10.1016/s0006-291x(88)81265-8. [DOI] [PubMed] [Google Scholar]
- Blough H. A., Pauwels R., De Clercq E., Cogniaux J., Sprecher-Goldberger S., Thiry L. Glycosylation inhibitors block the expression of LAV/HTLV-III (HIV) glycoproteins. Biochem Biophys Res Commun. 1986 Nov 26;141(1):33–38. doi: 10.1016/s0006-291x(86)80330-8. [DOI] [PubMed] [Google Scholar]
- Chen K. Y., Chang Z. F. A marked increase of fucosylation of glycoproteins in IMR-90 human diploid fibroblasts during senescence in vitro. Biochem Biophys Res Commun. 1987 Feb 13;142(3):767–774. doi: 10.1016/0006-291x(87)91480-x. [DOI] [PubMed] [Google Scholar]
- Chung S. W., Wolff L., Ruscetti S. Sequences responsible for the altered erythropoietin responsiveness in spleen focus-forming virus strain SFFVP-infected cells are localized to a 678-base-pair region at the 3' end of the envelope gene. J Virol. 1987 May;61(5):1661–1664. doi: 10.1128/jvi.61.5.1661-1664.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio V., Jacobs S., Romero P. A., Herscovics A. Effects of inhibitors of N-linked oligosaccharide processing on the biosynthesis and function of insulin and insulin-like growth factor-I receptors. J Biol Chem. 1988 Apr 15;263(11):5436–5445. [PubMed] [Google Scholar]
- Elbein A. D., Szumilo T., Sanford B. A., Sharpless K. B., Adams C. Effect of isomers of swainsonine on glycosidase activity and glycoprotein processing. Biochemistry. 1987 May 5;26(9):2502–2510. doi: 10.1021/bi00383a015. [DOI] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. Carbohydrate composition of the membrane glycoprotein of vesicular stomatitis virus grown in four mammalian cell lines. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4011–4014. doi: 10.1073/pnas.71.10.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foddy L., Hughes R. C. Assembly of asparagine-linked oligosaccharides in baby hamster kidney cells treated with castanospermine, an inhibitor of processing glucosidases. Eur J Biochem. 1988 Aug 1;175(2):291–299. doi: 10.1111/j.1432-1033.1988.tb14196.x. [DOI] [PubMed] [Google Scholar]
- Grant C. K., Ernisse B. J., Jarrett O., Jones F. R. Feline leukemia virus envelope gp70 of subgroups B and C defined by monoclonal antibodies with cytotoxic and neutralizing functions. J Immunol. 1983 Dec;131(6):3042–3048. [PubMed] [Google Scholar]
- Gruters R. A., Neefjes J. J., Tersmette M., de Goede R. E., Tulp A., Huisman H. G., Miedema F., Ploegh H. L. Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature. 1987 Nov 5;330(6143):74–77. doi: 10.1038/330074a0. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Mullins J. I., Quackenbush S. L., Gasper P. W. Experimental transmission and pathogenesis of immunodeficiency syndrome in cats. Blood. 1987 Dec;70(6):1880–1892. [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- Kaushal G. P., Pan Y. T., Tropea J. E., Mitchell M., Liu P., Elbein A. D. Selective inhibition of glycoprotein-processing enzymes. Differential inhibition of glucosidases I and II in cell culture. J Biol Chem. 1988 Nov 25;263(33):17278–17283. [PubMed] [Google Scholar]
- Kaushansky K., O'Hara P. J., Hart C. E., Forstrom J. W., Hagen F. S. Role of carbohydrate in the function of human granulocyte-macrophage colony-stimulating factor. Biochemistry. 1987 Jul 28;26(15):4861–4867. doi: 10.1021/bi00389a038. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Zeng Y. Pleiotropic resistance to glycoprotein processing inhibitors in Chinese hamster ovary cells. The role of a novel mutation in the asparagine-linked glycosylation pathway. J Biol Chem. 1989 Jan 25;264(3):1584–1593. [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. J Biol Chem. 1988 Apr 25;263(12):5955–5960. [PubMed] [Google Scholar]
- Machida C. A., Bestwick R. K., Boswell B. A., Kabat D. Role of a membrane glycoprotein in Friend virus-induced erythroleukemia: studies of mutant and revertant viruses. Virology. 1985 Jul 15;144(1):158–172. doi: 10.1016/0042-6822(85)90314-9. [DOI] [PubMed] [Google Scholar]
- McDowell W., Schwarz R. T. Dissecting glycoprotein biosynthesis by the use of specific inhibitors. Biochimie. 1988 Nov;70(11):1535–1549. doi: 10.1016/0300-9084(88)90290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Parekh B., Orrego A., Issel C. J. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J Biol Chem. 1984 Aug 25;259(16):10539–10544. [PubMed] [Google Scholar]
- Mullins J. I., Chen C. S., Hoover E. A. Disease-specific and tissue-specific production of unintegrated feline leukaemia virus variant DNA in feline AIDS. Nature. 1986 Jan 23;319(6051):333–336. doi: 10.1038/319333a0. [DOI] [PubMed] [Google Scholar]
- Mullins J. I., Hoover E. A., Overbaugh J., Quackenbush S. L., Donahue P. R., Poss M. L. FeLV-FAIDS-induced immunodeficiency syndrome in cats. Vet Immunol Immunopathol. 1989 May;21(1):25–37. doi: 10.1016/0165-2427(89)90127-x. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Alterations of cell-surface carbohydrates during differentiation and development. Biochimie. 1988 Nov;70(11):1587–1596. doi: 10.1016/0300-9084(88)90294-5. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Host cell- and virus strain-dependent differences in oligosaccharides of hemagglutinin glycoproteins of influenza A viruses. Virology. 1979 May;95(1):8–23. doi: 10.1016/0042-6822(79)90397-0. [DOI] [PubMed] [Google Scholar]
- Ogier-Denis E., Codogno P., Chantret I., Trugnan G. The processing of asparagine-linked oligosaccharides in HT-29 cells is a function of their state of enterocytic differentiation. An accumulation of Man9,8-GlcNAc2-Asn species is indicative of an impaired N-glycan trimming in undifferentiated cells. J Biol Chem. 1988 May 5;263(13):6031–6037. [PubMed] [Google Scholar]
- Overbaugh J., Donahue P. R., Quackenbush S. L., Hoover E. A., Mullins J. I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988 Feb 19;239(4842):906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Tse A. G., Dwek R. A., Williams A. F., Rademacher T. W. Tissue-specific N-glycosylation, site-specific oligosaccharide patterns and lentil lectin recognition of rat Thy-1. EMBO J. 1987 May;6(5):1233–1244. doi: 10.1002/j.1460-2075.1987.tb02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
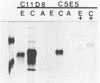
- Poss M. L., Mullins J. I., Hoover E. A. Posttranslational modifications distinguish the envelope glycoprotein of the immunodeficiency disease-inducing feline leukemia virus retrovirus. J Virol. 1989 Jan;63(1):189–195. doi: 10.1128/jvi.63.1.189-195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L. D., Bause E., Legler G., Molyneux R. J., Hart G. W. Influence of asparagine-linked oligosaccharides on tumor cell recognition in the mixed lymphocyte reaction. J Immunol. 1985 Jul;135(1):714–724. [PubMed] [Google Scholar]
- Powell L. D., Smith K., Hart G. W. Site specific glycosylation patterns of H-2K: effects of allelic polymorphism and mitogenic stimulation. J Immunol. 1987 Aug 15;139(4):1206–1213. [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Dornsife R. E., Mullins J. I. Pathogenic and host range determinants of the feline aplastic anemia retrovirus. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2758–2762. doi: 10.1073/pnas.85.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P. A., Datema R., Schwarz R. T. N-methyl-1-deoxynojirimycin, a novel inhibitor of glycoprotein processing, and its effect on fowl plague virus maturation. Virology. 1983 Oct 15;130(1):238–242. doi: 10.1016/0042-6822(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Rosner M. R., Grinna L. S., Robbins P. W. Differences in glycosylation patterns of closely related murine leukemia viruses. Proc Natl Acad Sci U S A. 1980 Jan;77(1):67–71. doi: 10.1073/pnas.77.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. L., Dalrymple J. M., Johnston R. E. Sindbis virus mutations which coordinately affect glycoprotein processing, penetration, and virulence in mice. J Virol. 1989 Apr;63(4):1619–1629. doi: 10.1128/jvi.63.4.1619-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinovich O., Montelaro R. C. Comparison of glycoproteins by two-dimensional mapping of glycosylated peptides. Anal Biochem. 1986 Aug 15;157(1):19–27. doi: 10.1016/0003-2697(86)90190-9. [DOI] [PubMed] [Google Scholar]
- Shiroishi T., Evans G. A., Appella E., Ozato K. In vitro mutagenesis of a mouse MHC class I gene for the examination of structure-function relationships. J Immunol. 1985 Jan;134(1):623–629. [PubMed] [Google Scholar]
- Snyder H. W., Jr, Hardy W. D., Jr, Zuckerman E. E., Fleissner E. Characterisation of a tumour-specific antigen on the surface of feline lymphosarcoma cells. Nature. 1978 Oct 19;275(5681):656–658. doi: 10.1038/275656a0. [DOI] [PubMed] [Google Scholar]
- Soderquist A. M., Carpenter G. Glycosylation of the epidermal growth factor receptor in A-431 cells. The contribution of carbohydrate to receptor function. J Biol Chem. 1984 Oct 25;259(20):12586–12594. [PubMed] [Google Scholar]
- Szurek P. F., Yuen P. H., Jerzy R., Wong P. K. Identification of point mutations in the envelope gene of Moloney murine leukemia virus TB temperature-sensitive paralytogenic mutant ts1: molecular determinants for neurovirulence. J Virol. 1988 Jan;62(1):357–360. doi: 10.1128/jvi.62.1.357-360.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. D., Kowalski M., Goh W. C., Kozarsky K., Krieger M., Rosen C., Rohrschneider L., Haseltine W. A., Sodroski J. Inhibition of human immunodeficiency virus syncytium formation and virus replication by castanospermine. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8120–8124. doi: 10.1073/pnas.84.22.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. The spleen focus-forming virus (SFFV) envelope gene, when introduced into mice in the absence of other SFFV genes, induces acute erythroleukemia. J Virol. 1988 Jun;62(6):2158–2163. doi: 10.1128/jvi.62.6.2158-2163.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen P. H., Tzeng E., Knupp C., Wong P. K. The neurovirulent determinants of ts1, a paralytogenic mutant of Moloney murine leukemia virus TB, are localized in at least two functionally distinct regions of the genome. J Virol. 1986 Jul;59(1):59–65. doi: 10.1128/jvi.59.1.59-65.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]