Abstract
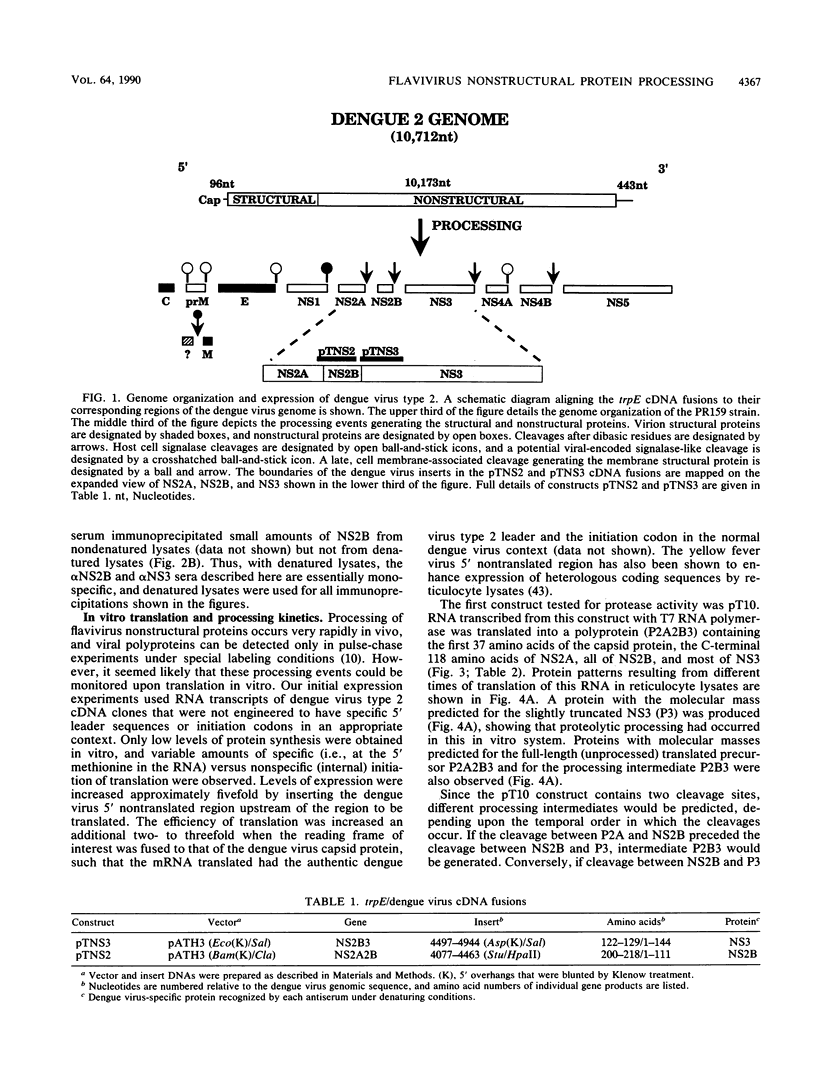
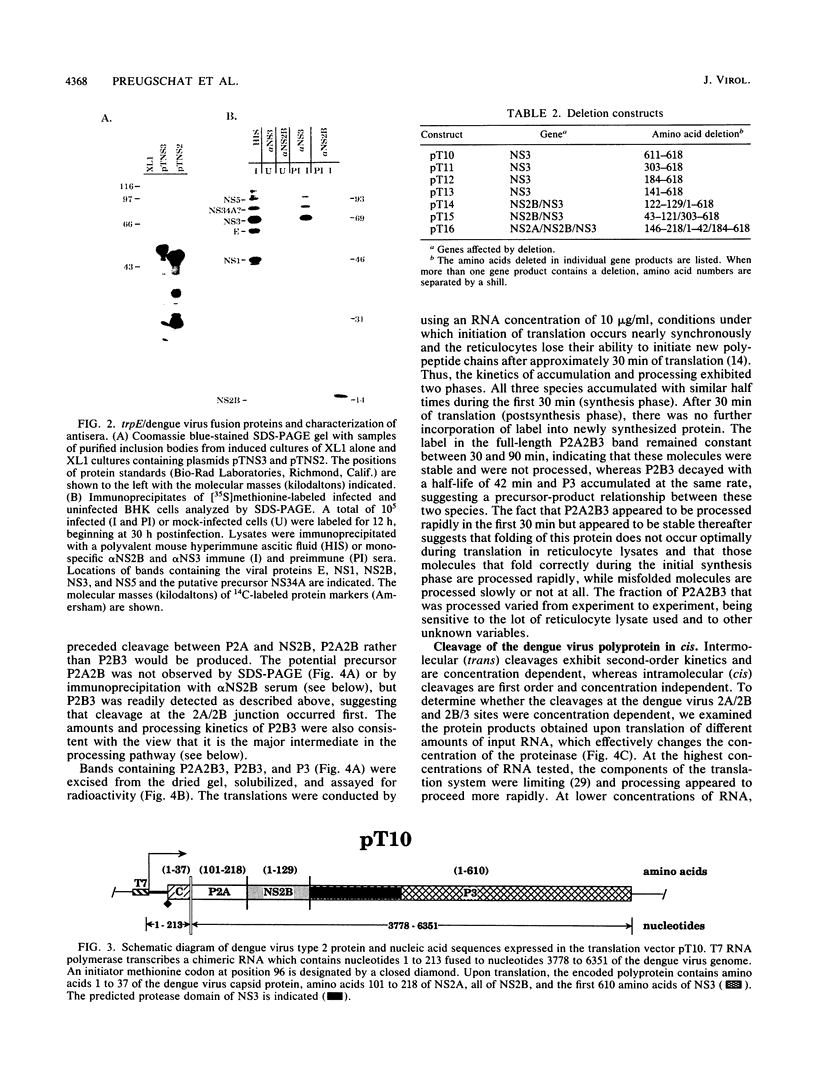
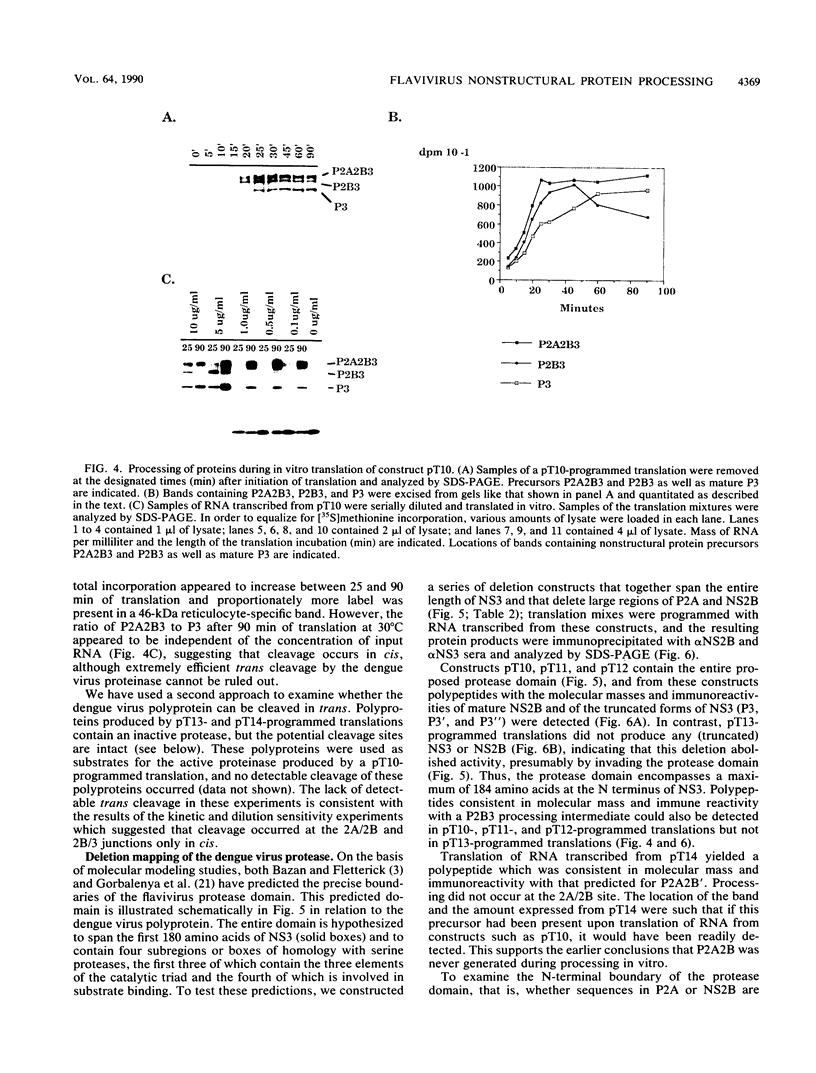
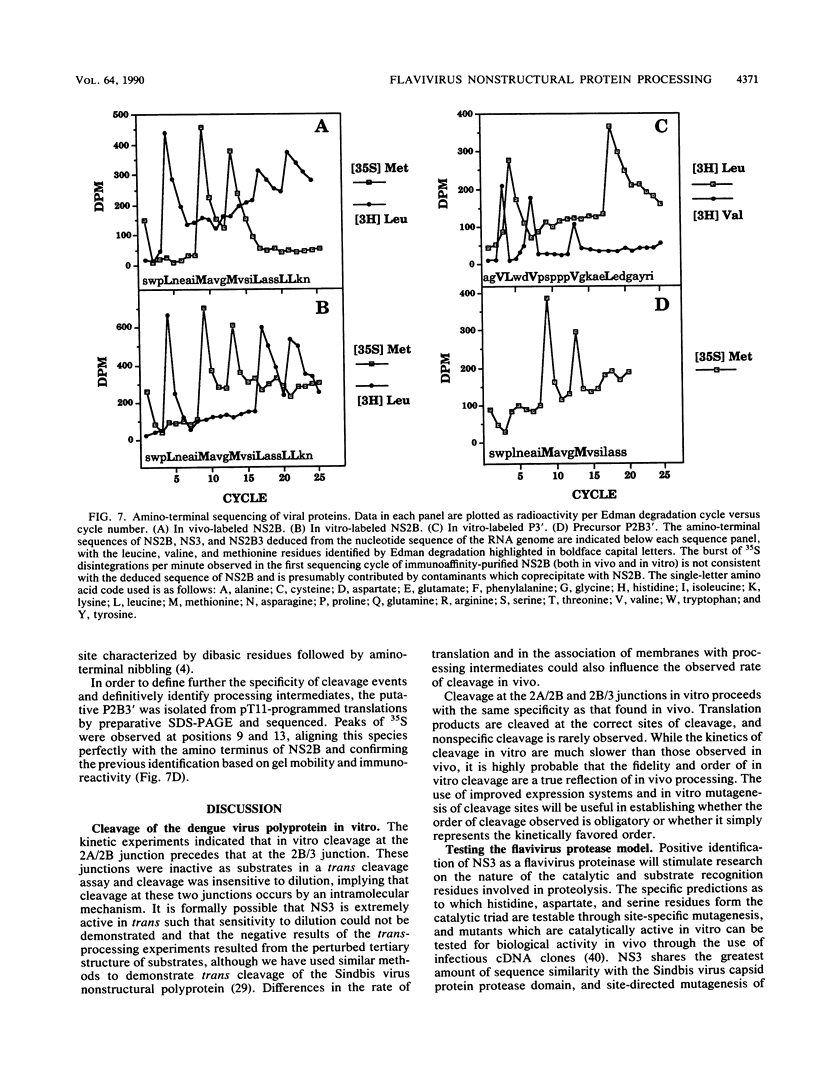
We have tested the hypothesis that the flavivirus nonstructural protein NS3 is a viral proteinase that generates the termini of several nonstructural proteins by using an efficient in vitro expression system and monospecific antisera directed against the nonstructural proteins NS2B and NS3. A series of cDNA constructs was transcribed by using T7 RNA polymerase, and the RNA was translated in reticulocyte lysates. The resulting protein patterns indicated that proteolytic processing occurred in vitro to generate NS2B and NS3. The amino termini of NS2B and NS3 produced in vitro were found to be the same as the termini of NS2B and NS3 isolated from infected cells. Deletion analysis of cDNA constructs localized the protease domain within NS3 to the first 184 amino acids but did not eliminate the possibility that sequences within NS2B were also required for proper cleavage. Kinetic analysis of processing events in vitro and experiments to examine the sensitivity of processing to dilution suggested that an intramolecular cleavage between NS2A and NS2B preceded an intramolecular cleavage between NS2B and NS3. The data from these expression experiments confirm that NS3 is the viral proteinase responsible for cleavage events generating the amino termini of NS2B and NS3 and presumably for cleavages generating the termini of NS4A and NS5 as well.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft W. H., Scott R. M., Eckels K. H., Hoke C. H., Jr, Simms T. E., Jesrani K. D., Summers P. L., Dubois D. R., Tsoulos D., Russell P. K. Dengue virus type 2 vaccine: reactogenicity and immunogenicity in soldiers. J Infect Dis. 1984 Jun;149(6):1005–1010. doi: 10.1093/infdis/149.6.1005. [DOI] [PubMed] [Google Scholar]
- Bancroft W. H., Top F. H., Jr, Eckels K. H., Anderson J. H., Jr, McCown J. M., Russell P. K. Dengue-2 vaccine: virological, immunological, and clinical responses of six yellow fever-immune recipients. Infect Immun. 1981 Feb;31(2):698–703. doi: 10.1128/iai.31.2.698-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F., Fletterick R. J. Detection of a trypsin-like serine protease domain in flaviviruses and pestiviruses. Virology. 1989 Aug;171(2):637–639. doi: 10.1016/0042-6822(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Biedrzycka A., Cauchi M. R., Bartholomeusz A., Gorman J. J., Wright P. J. Characterization of protease cleavage sites involved in the formation of the envelope glycoprotein and three non-structural proteins of dengue virus type 2, New Guinea C strain. J Gen Virol. 1987 May;68(Pt 5):1317–1326. doi: 10.1099/0022-1317-68-5-1317. [DOI] [PubMed] [Google Scholar]
- Boege U., Cygler M., Wengler G., Dumas P., Tsao J., Luo M., Smith T. J., Rossmann M. G. Sindbis virus core protein crystals. J Mol Biol. 1989 Jul 5;208(1):79–82. doi: 10.1016/0022-2836(89)90089-2. [DOI] [PubMed] [Google Scholar]
- Calisher C. H., Karabatsos N., Dalrymple J. M., Shope R. E., Porterfield J. S., Westaway E. G., Brandt W. E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol. 1989 Jan;70(Pt 1):37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- Calisher C. H., Shope R. E., Brandt W., Casals J., Karabatsos N., Murphy F. A., Tesh R. B., Wiebe M. E. Proposed antigenic classification of registered arboviruses I. Togaviridae, Alphavirus. Intervirology. 1980;14(5-6):229–232. doi: 10.1159/000149190. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., McCourt D. W., Rice C. M. Yellow fever virus proteins NS2A, NS2B, and NS4B: identification and partial N-terminal amino acid sequence analysis. Virology. 1989 Mar;169(1):100–109. doi: 10.1016/0042-6822(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Cleaves G. R., Dubin D. T. Methylation status of intracellular dengue type 2 40 S RNA. Virology. 1979 Jul 15;96(1):159–165. doi: 10.1016/0042-6822(79)90181-8. [DOI] [PubMed] [Google Scholar]
- Cleaves G. R. Identification of dengue type 2 virus-specific high molecular weight proteins in virus-infected BHK cells. J Gen Virol. 1985 Dec;66(Pt 12):2767–2771. doi: 10.1099/0022-1317-66-12-2767. [DOI] [PubMed] [Google Scholar]
- Coia G., Parker M. D., Speight G., Byrne M. E., Westaway E. G. Nucleotide and complete amino acid sequences of Kunjin virus: definitive gene order and characteristics of the virus-specified proteins. J Gen Virol. 1988 Jan;69(Pt 1):1–21. doi: 10.1099/0022-1317-69-1-1. [DOI] [PubMed] [Google Scholar]
- Condreay L. D., Adams R. H., Edwards J., Brown D. T. Effect of actinomycin D and cycloheximide on replication of Sindbis virus in Aedes albopictus (mosquito) cells. J Virol. 1988 Aug;62(8):2629–2635. doi: 10.1128/jvi.62.8.2629-2635.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M. C., Jackson R. J. On the fidelity of mRNA translation in the nuclease-treated rabbit reticulocyte lysate system. Nucleic Acids Res. 1989 Apr 25;17(8):3129–3144. doi: 10.1093/nar/17.8.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubel V., Kinney R. M., Trent D. W. Nucleotide sequence and deduced amino acid sequence of the nonstructural proteins of dengue type 2 virus, Jamaica genotype: comparative analysis of the full-length genome. Virology. 1988 Jul;165(1):234–244. doi: 10.1016/0042-6822(88)90677-0. [DOI] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Eckels K. H., Harrison V. R., Summers P. L., Russell P. K. Dengue-2 vaccine: preparation from a small-plaque virus clone. Infect Immun. 1980 Jan;27(1):175–180. doi: 10.1128/iai.27.1.175-180.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenkranz N. J., Ventura A. K., Cuadrado R. R., Pond W. L., Porter J. E. Pandemic dengue in Caribbean countries and the southern United States--past, present and potential problems. N Engl J Med. 1971 Dec 23;285(26):1460–1469. doi: 10.1056/NEJM197112232852606. [DOI] [PubMed] [Google Scholar]
- Falgout B., Chanock R., Lai C. J. Proper processing of dengue virus nonstructural glycoprotein NS1 requires the N-terminal hydrophobic signal sequence and the downstream nonstructural protein NS2a. J Virol. 1989 May;63(5):1852–1860. doi: 10.1128/jvi.63.5.1852-1860.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Blinov V. M., Koonin E. V. Cysteine proteases of positive strand RNA viruses and chymotrypsin-like serine proteases. A distinct protein superfamily with a common structural fold. FEBS Lett. 1989 Jan 30;243(2):103–114. doi: 10.1016/0014-5793(89)80109-7. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Koonin E. V., Blinov V. M. N-terminal domains of putative helicases of flavi- and pestiviruses may be serine proteases. Nucleic Acids Res. 1989 May 25;17(10):3889–3897. doi: 10.1093/nar/17.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. A conserved NTP-motif in putative helicases. Nature. 1988 May 5;333(6168):22–22. doi: 10.1038/333022a0. [DOI] [PubMed] [Google Scholar]
- Gruenberg A., Woo W. S., Biedrzycka A., Wright P. J. Partial nucleotide sequence and deduced amino acid sequence of the structural proteins of dengue virus type 2, New Guinea C and PUO-218 strains. J Gen Virol. 1988 Jun;69(Pt 6):1391–1398. doi: 10.1099/0022-1317-69-6-1391. [DOI] [PubMed] [Google Scholar]
- Gubler D. J. Aedes aegypti and Aedes aegypti-borne disease control in the 1990s: top down or bottom up. Charles Franklin Craig Lecture. Am J Trop Med Hyg. 1989 Jun;40(6):571–578. doi: 10.4269/ajtmh.1989.40.571. [DOI] [PubMed] [Google Scholar]
- Hahn C. S., Strauss E. G., Strauss J. H. Sequence analysis of three Sindbis virus mutants temperature-sensitive in the capsid protein autoprotease. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4648–4652. doi: 10.1073/pnas.82.14.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Strauss J. H. Site-directed mutagenesis of the proposed catalytic amino acids of the Sindbis virus capsid protein autoprotease. J Virol. 1990 Jun;64(6):3069–3073. doi: 10.1128/jvi.64.6.3069-3073.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Galler R., Hunkapiller T., Dalrymple J. M., Strauss J. H., Strauss E. G. Nucleotide sequence of dengue 2 RNA and comparison of the encoded proteins with those of other flaviviruses. Virology. 1988 Jan;162(1):167–180. doi: 10.1016/0042-6822(88)90406-0. [DOI] [PubMed] [Google Scholar]
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of Sindbis virus: study of the kinetics in vivo by using monospecific antibodies. J Virol. 1988 Mar;62(3):998–1007. doi: 10.1128/jvi.62.3.998-1007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of sindbis virus: nonstructural proteinase is in the C-terminal half of nsP2 and functions both in cis and in trans. J Virol. 1989 Nov;63(11):4653–4664. doi: 10.1128/jvi.63.11.4653-4664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markoff L. In vitro processing of dengue virus structural proteins: cleavage of the pre-membrane protein. J Virol. 1989 Aug;63(8):3345–3352. doi: 10.1128/jvi.63.8.3345-3352.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nowak T., Färber P. M., Wengler G., Wengler G. Analyses of the terminal sequences of West Nile virus structural proteins and of the in vitro translation of these proteins allow the proposal of a complete scheme of the proteolytic cleavages involved in their synthesis. Virology. 1989 Apr;169(2):365–376. doi: 10.1016/0042-6822(89)90162-1. [DOI] [PubMed] [Google Scholar]
- Ozden S., Poirier B. Dengue virus induced polypeptide synthesis. Brief report. Arch Virol. 1985;85(1-2):129–137. doi: 10.1007/BF01317012. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Aebersold R., Teplow D. B., Pata J., Bell J. R., Vorndam A. V., Trent D. W., Brandriss M. W., Schlesinger J. J., Strauss J. H. Partial N-terminal amino acid sequences of three nonstructural proteins of two flaviviruses. Virology. 1986 May;151(1):1–9. doi: 10.1016/0042-6822(86)90098-x. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Grakoui A., Galler R., Chambers T. J. Transcription of infectious yellow fever RNA from full-length cDNA templates produced by in vitro ligation. New Biol. 1989 Dec;1(3):285–296. [PubMed] [Google Scholar]
- Rice C. M., Lenches E. M., Eddy S. R., Shin S. J., Sheets R. L., Strauss J. H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985 Aug 23;229(4715):726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Levis R., Strauss J. H., Huang H. V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Linares A., Bouloy M., Girard M., Cahour A. Modulations of the in vitro translational efficiencies of Yellow Fever virus mRNAs: interactions between coding and noncoding regions. Nucleic Acids Res. 1989 Apr 11;17(7):2463–2476. doi: 10.1093/nar/17.7.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Linares A., Cahour A., Després P., Girard M., Bouloy M. Processing of yellow fever virus polyprotein: role of cellular proteases in maturation of the structural proteins. J Virol. 1989 Oct;63(10):4199–4209. doi: 10.1128/jvi.63.10.4199-4209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmayr H. G., Nogueira R. M., Travassos da Rosa A. P. An outbreak of dengue virus at Rio de Janeiro--1986. Mem Inst Oswaldo Cruz. 1986 Apr-Jun;81(2):245–246. doi: 10.1590/s0074-02761986000200019. [DOI] [PubMed] [Google Scholar]
- Scott R. M., Eckels K. H., Bancroft W. H., Summers P. L., McCown J. M., Anderson J. H., Russell P. K. Dengue 2 vaccine: dose response in volunteers in relation to yellow fever immune status. J Infect Dis. 1983 Dec;148(6):1055–1060. doi: 10.1093/infdis/148.6.1055. [DOI] [PubMed] [Google Scholar]
- Smith G. W., Wright P. J. Synthesis of proteins and glycoproteins in dengue type 2 virus-infected vero and Aedes albopictus cells. J Gen Virol. 1985 Mar;66(Pt 3):559–571. doi: 10.1099/0022-1317-66-3-559. [DOI] [PubMed] [Google Scholar]
- Speight G., Coia G., Parker M. D., Westaway E. G. Gene mapping and positive identification of the non-structural proteins NS2A, NS2B, NS3, NS4B and NS5 of the flavivirus Kunjin and their cleavage sites. J Gen Virol. 1988 Jan;69(Pt 1):23–34. doi: 10.1099/0022-1317-69-1-23. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Beato M., Wengler G. In vitro translation of 42 S virus-specific RNA from cells infected with the flavivirus West Nile virus. Virology. 1979 Jul 30;96(2):516–529. doi: 10.1016/0042-6822(79)90108-9. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Gross H. J. Studies on virus-specific nucleic acids synthesized in vertebrate and mosquito cells infected with flaviviruses. Virology. 1978 Sep;89(2):423–437. doi: 10.1016/0042-6822(78)90185-x. [DOI] [PubMed] [Google Scholar]
- Wright P. J., Cauchi M. R., Ng M. L. Definition of the carboxy termini of the three glycoproteins specified by dengue virus type 2. Virology. 1989 Jul;171(1):61–67. doi: 10.1016/0042-6822(89)90510-2. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]