Abstract
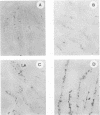
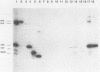
Severe combined immunodeficient (SCID) mice lack both functional T and B cells. These mice develop chronic rotavirus infection following an oral inoculation with the epizootic diarrhea of infant mice (EDIM) rotavirus. Reconstitution of rotavirus-infected SCID mice with T lymphocytes from immunocompetent mice allows an evaluation of a role of T-cell-mediated immunity in clearing chronic rotavirus infection. Complete rotavirus clearance was demonstrated in C.B-17/scid mice 7 to 9 days after the transfer of immune CD8+ splenic T lymphocytes from histocompatible BALB/c mice previously immunized intraperitoneally with the EDIM-w strain of murine rotavirus. The virus clearance mediated by T-cell transfer was restricted to H-2d-bearing T cells and occurred in the absence of rotavirus-specific antibody as determined by enzyme-linked immunosorbent assay, neutralization, immunohistochemistry, and radioimmunoprecipitation. Temporary clearance of rotavirus was observed after the transfer of immune CD8+ T cells isolated from the intestinal mucosa (intraepithelial lymphocytes [IELs]) or the spleens of BALB/c mice previously infected with EDIM by the oral route. Chronic virus shedding was transiently eliminated 7 to 11 days after spleen cell transfer and 11 to 12 days after IEL transfer. However, recurrence of rotavirus infection was detected 1 to 8 days later in all but one SCID recipient receiving cells from orally immunized donors. The viral clearance was mediated by IELs that were both Thy1+ and CD8+. These data demonstrated that the clearance of chronic rotavirus infection in SCID mice can be mediated by immune CD8+ T lymphocytes and that this clearance can occur in the absence of virus-specific antibodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiba S., Yokoyama T., Nakata S., Morita Y., Urasawa T., Taniguchi K., Urasawa S., Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986 Aug 23;2(8504):417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- Davidson G. P., Barnes G. L. Structural and functional abnormalities of the small intestine in infants and young children with rotavirus enteritis. Acta Paediatr Scand. 1979 Mar;68(2):181–186. doi: 10.1111/j.1651-2227.1979.tb04986.x. [DOI] [PubMed] [Google Scholar]
- Dharakul T., Riepenhoff-Talty M., Albini B., Ogra P. L. Distribution of rotavirus antigen in intestinal lymphoid tissues: potential role in development of the mucosal immune response to rotavirus. Clin Exp Immunol. 1988 Oct;74(1):14–19. [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K., Pollack S. B., Bosma M. J., Phillips R. A. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (scid). J Immunol. 1985 Jun;134(6):3798–3801. [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Vo P. T., Jones R. Cultivation and characterization of three strains of murine rotavirus. J Virol. 1986 Feb;57(2):585–590. doi: 10.1128/jvi.57.2.585-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood K., Lund J. C., Coulson B. S., Hudson I. L., Bishop R. F., Barnes G. L. Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol. 1988 Apr;26(4):732–738. doi: 10.1128/jcm.26.4.732-738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. R. Ontogeny of the Thy-1-, Lyt-2+ murine intestinal intraepithelial lymphocyte. Characterization of a unique population of thymus-independent cytotoxic effector cells in the intestinal mucosa. J Exp Med. 1986 Jul 1;164(1):309–314. doi: 10.1084/jem.164.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984 Jul;51(1):208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonsky G. A., Johnson J. P., Winkelstein J. A., Yolken R. H. Oral administration of human serum immunoglobulin in immunodeficient patients with viral gastroenteritis. A pharmacokinetic and functional analysis. J Clin Invest. 1985 Dec;76(6):2362–2367. doi: 10.1172/JCI112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. M., Offit P. A., Vo P. T., Mackow E. R., Benfield D. A., Shaw R. D., Padilla-Noriega L., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to the heterotypic neutralization domain of VP7 and the VP8 fragment of VP4. J Clin Microbiol. 1989 Apr;27(4):780–782. doi: 10.1128/jcm.27.4.780-782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985 Apr;54(1):58–64. doi: 10.1128/jvi.54.1.58-64.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes appear at the intestinal mucosal surface after rotavirus infection. J Virol. 1989 Aug;63(8):3507–3512. doi: 10.1128/jvi.63.8.3507-3512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes cross-react with target cells infected with different rotavirus serotypes. J Virol. 1988 Jan;62(1):127–131. doi: 10.1128/jvi.62.1.127-131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott D. M., Tait C., MacKenzie S., Mowat A. M., Davies M. D., Micklem H. S. Analysis of the effector functions of different populations of mucosal lymphocytes. Ann N Y Acad Sci. 1983 Jun 30;409:307–320. doi: 10.1111/j.1749-6632.1983.tb26879.x. [DOI] [PubMed] [Google Scholar]
- Petit A., Ernst P. B., Befus A. D., Clark D. A., Rosenthal K. L., Ishizaka T., Bienenstock J. Murine intestinal intraepithelial lymphocytes I. Relationship of a novel Thy-1-,Lyt-1-,Lyt-2+, granulated subpopulation to natural killer cells and mast cells. Eur J Immunol. 1985 Mar;15(3):211–215. doi: 10.1002/eji.1830150302. [DOI] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Dharakul T., Kowalski E., Michalak S., Ogra P. L. Persistent rotavirus infection in mice with severe combined immunodeficiency. J Virol. 1987 Oct;61(10):3345–3348. doi: 10.1128/jvi.61.10.3345-3348.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulsbury F. T., Winkelstein J. A., Yolken R. H. Chronic rotavirus infection in immunodeficiency. J Pediatr. 1980 Jul;97(1):61–65. doi: 10.1016/s0022-3476(80)80131-4. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Stoner-Ma D. L., Estes M. K., Greenberg H. B. Specific enzyme-linked immunoassay for rotavirus serotypes 1 and 3. J Clin Microbiol. 1985 Aug;22(2):286–291. doi: 10.1128/jcm.22.2.286-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. L., Cukor G., Blacklow N. R., Dambrauskas R., Trier J. S. Susceptibility of mice to rotavirus infection: effects of age and administration of corticosteroids. Infect Immun. 1981 Aug;33(2):565–574. doi: 10.1128/iai.33.2.565-574.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]