Abstract
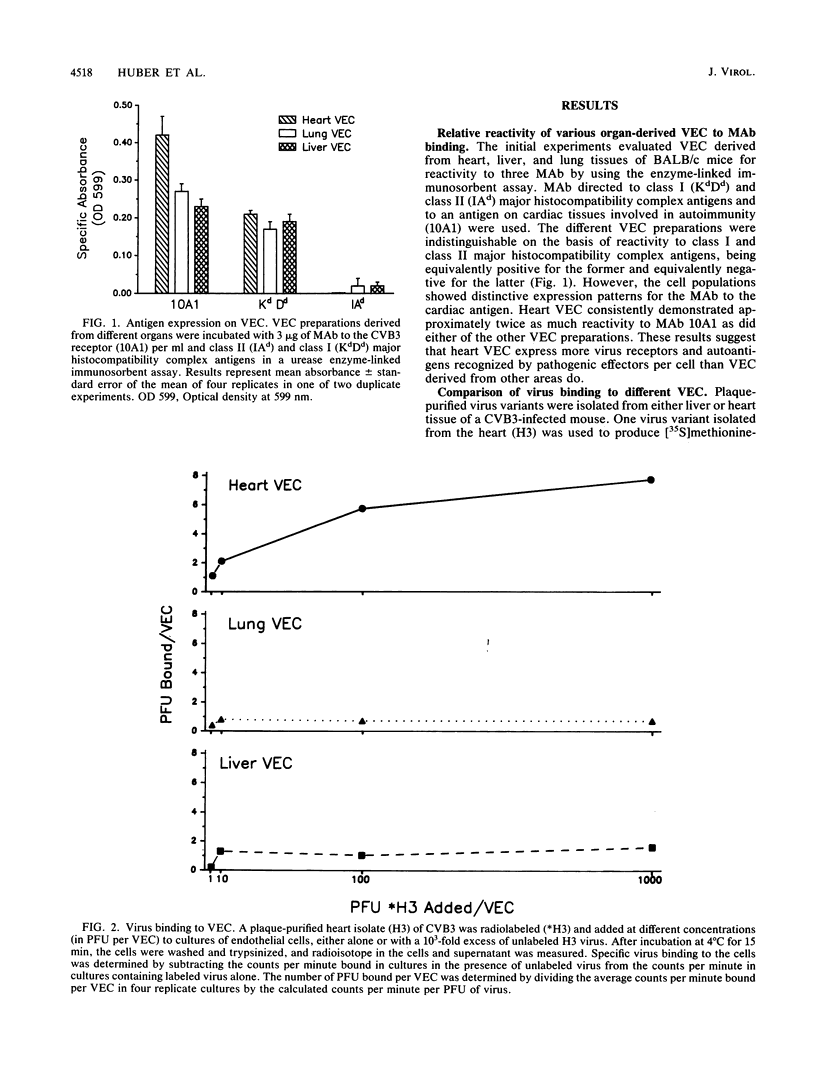
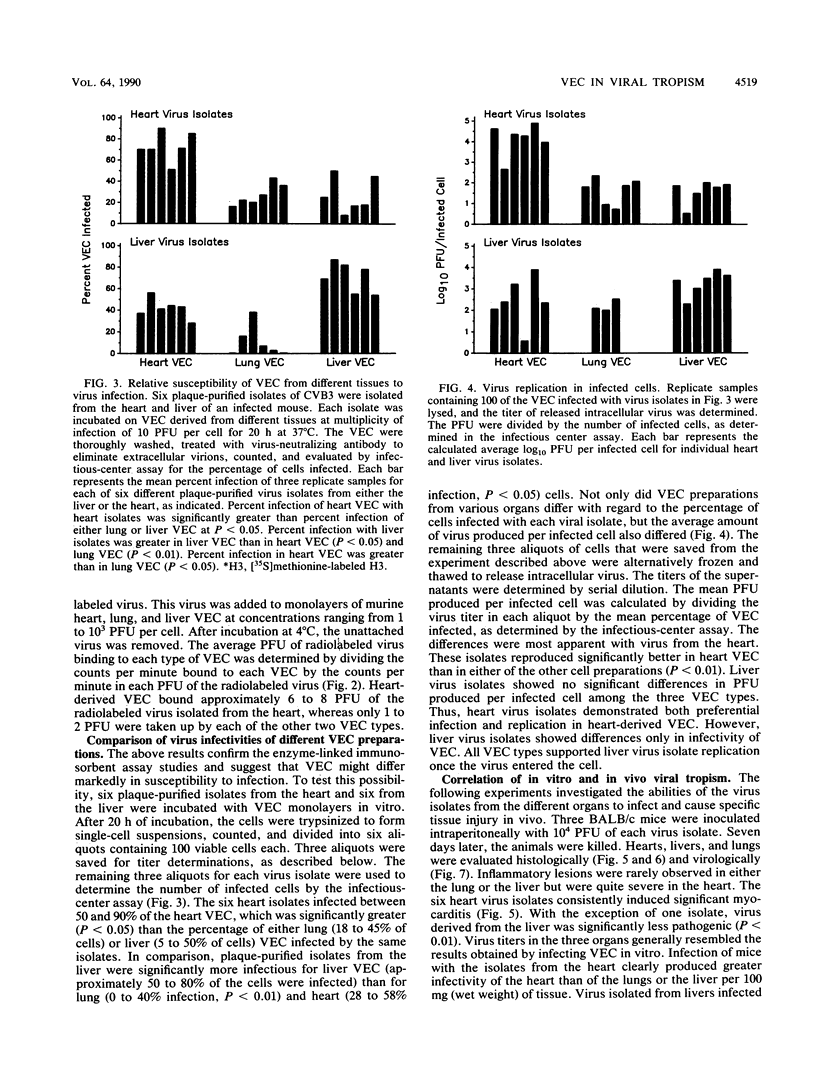
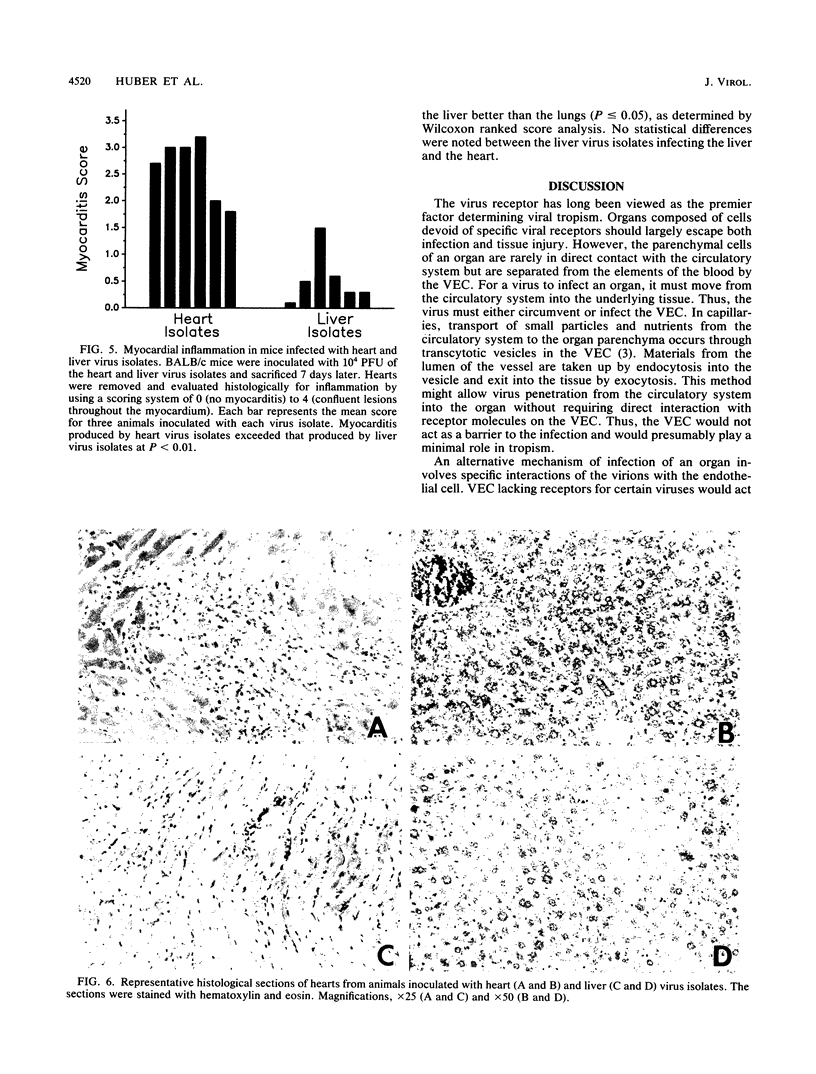
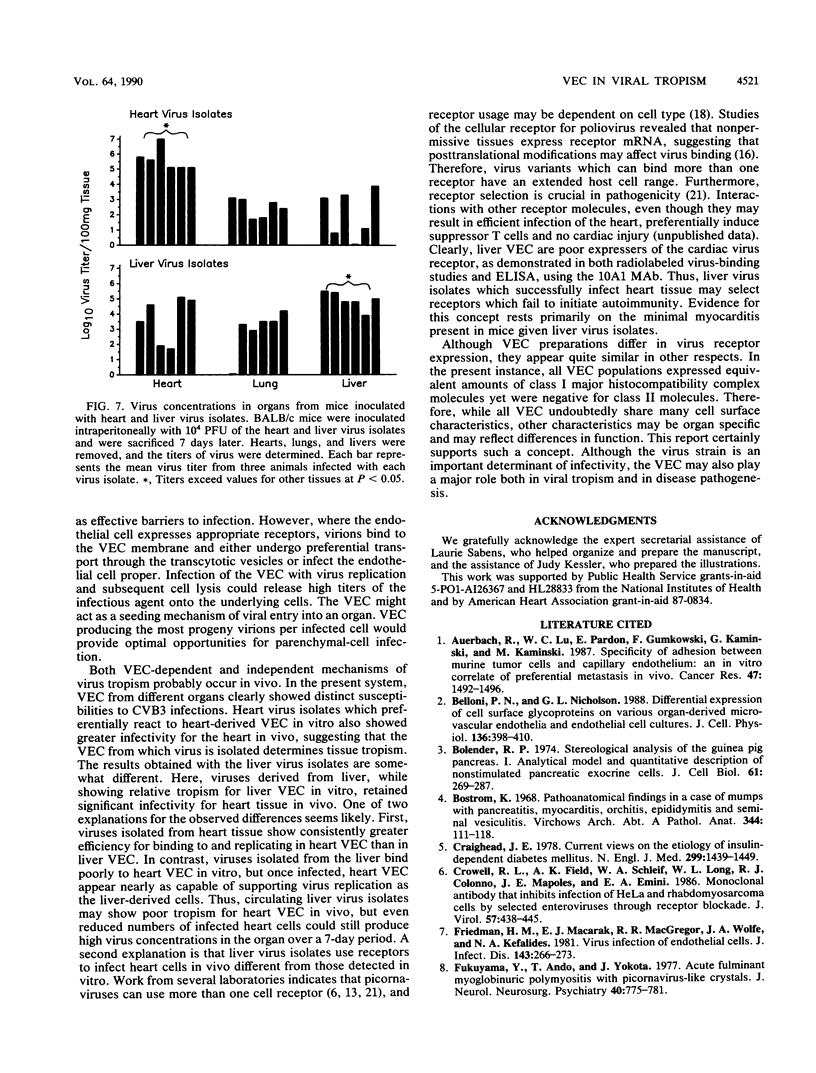
Six plaque-purified virus isolates were obtained from liver and heart tissues of a DBA/2 mouse infected 7 days earlier with 10(4) PFU of coxsackievirus group B type 3. Each virus isolate was assayed in vitro for infectivity to vascular endothelial cells (VEC) of the liver, lungs, and heart. Both the percentage of VEC infected and the mean progeny PFU produced per infected VEC were determined. Virus isolates from the heart showed greater infectivity and replication in heart VEC than in VEC derived from either the liver or lungs. Similarly, virus isolated from the liver preferentially infected liver VEC. Virus receptor expression varied between VEC populations, as demonstrated by binding studies with a [35S]methionine-radiolabeled heart virus and by enzyme-linked immunoadsorption assay studies with a monoclonal antibody to the coxsackievirus group B type 3 receptor on heart tissue. Finally, the heart and liver virus isolates were injected (10(4) PFU) intraperitoneally into BALB/c mice. After 7 days, the animals were sacrificed, and the hearts, livers, and lungs were evaluated for tissue injury and virus concentrations. Viruses originally isolated from the heart preferentially infected the heart when reinjected into animals and caused severe myocarditis. Viruses originally derived from the liver most consistently reinfected the liver, although significant virus concentrations were also detected in the heart. The liver virus isolates, however, were incapable of causing myocarditis. Thus, selective tropism of viruses for particular organs in vivo corresponds to the ability of these isolates to infect VEC in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach R., Lu W. C., Pardon E., Gumkowski F., Kaminska G., Kaminski M. Specificity of adhesion between murine tumor cells and capillary endothelium: an in vitro correlate of preferential metastasis in vivo. Cancer Res. 1987 Mar 15;47(6):1492–1496. [PubMed] [Google Scholar]
- Belloni P. N., Nicolson G. L. Differential expression of cell surface glycoproteins on various organ-derived microvascular endothelia and endothelial cell cultures. J Cell Physiol. 1988 Sep;136(3):398–410. doi: 10.1002/jcp.1041360303. [DOI] [PubMed] [Google Scholar]
- Bolender R. P. Stereological analysis of the guinea pig pancreas. I. Analytical model and quantitative description of nonstimulated pancreatic exocrine cells. J Cell Biol. 1974 May;61(2):269–287. doi: 10.1083/jcb.61.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström K. Patho-anatomical findings in a case of mumps with pancreatitis, myocarditis, orchitis, epididymitis and seminal vesiculitis. Virchows Arch A Pathol Pathol Anat. 1968;344(1):111–118. [PubMed] [Google Scholar]
- Craighead J. E. Current views on the etiology of insulin-dependent diabetes mellitus. N Engl J Med. 1978 Dec 28;299(26):1439–1445. doi: 10.1056/NEJM197812282992605. [DOI] [PubMed] [Google Scholar]
- Crowell R. L., Field A. K., Schleif W. A., Long W. L., Colonno R. J., Mapoles J. E., Emini E. A. Monoclonal antibody that inhibits infection of HeLa and rhabdomyosarcoma cells by selected enteroviruses through receptor blockade. J Virol. 1986 Feb;57(2):438–445. doi: 10.1128/jvi.57.2.438-445.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Macarak E. J., MacGregor R. R., Wolfe J., Kefalides N. A. Virus infection of endothelial cells. J Infect Dis. 1981 Feb;143(2):266–273. doi: 10.1093/infdis/143.2.266. [DOI] [PubMed] [Google Scholar]
- Fukuyama Y., Ando T., Yokota J. Acute fulminant myoglobinuric polymyositis with picornavirus-like crystals. J Neurol Neurosurg Psychiatry. 1977 Aug;40(8):775–781. doi: 10.1136/jnnp.40.8.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumkowski F., Kaminska G., Kaminski M., Morrissey L. W., Auerbach R. Heterogeneity of mouse vascular endothelium. In vitro studies of lymphatic, large blood vessel and microvascular endothelial cells. Blood Vessels. 1987;24(1-2):11–23. [PubMed] [Google Scholar]
- HORSTMANN D. M. EPIDEMIOLOGY OF POLIOMYELITIS AND ALLIED DISEASES--1963. Yale J Biol Med. 1963 Aug;36:5–26. [PMC free article] [PubMed] [Google Scholar]
- Hirschman S. Z., Hammer G. S. Coxsackie virus myopericarditis. A microbiological and clinical review. Am J Cardiol. 1974 Aug;34(2):224–232. doi: 10.1016/0002-9149(74)90201-x. [DOI] [PubMed] [Google Scholar]
- Hsu K. H., Crowell R. L. Characterization of a YAC-1 mouse cell receptor for group B coxsackieviruses. J Virol. 1989 Jul;63(7):3105–3108. doi: 10.1128/jvi.63.7.3105-3108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu K. H., Lonberg-Holm K., Alstein B., Crowell R. L. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J Virol. 1988 May;62(5):1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984 Jul;116(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Kumar S., West D. C., Ager A. Heterogeneity in endothelial cells from large vessels and microvessels. Differentiation. 1987;36(1):57–70. doi: 10.1111/j.1432-0436.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Owman C., Hardebo J. E. Functional heterogeneity of the cerebrovascular endothelium. Brain Behav Evol. 1988;32(2):65–75. doi: 10.1159/000116534. [DOI] [PubMed] [Google Scholar]
- Reagan K. J., Goldberg B., Crowell R. L. Altered receptor specificity of coxsackievirus B3 after growth in rhabdomyosarcoma cells. J Virol. 1984 Mar;49(3):635–640. doi: 10.1128/jvi.49.3.635-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram S., Steinman L. Postinfectious and postvaccinial encephalomyelitis. Neurol Clin. 1984 May;2(2):341–353. [PubMed] [Google Scholar]
- Turner R. R., Beckstead J. H., Warnke R. A., Wood G. S. Endothelial cell phenotypic diversity. In situ demonstration of immunologic and enzymatic heterogeneity that correlates with specific morphologic subtypes. Am J Clin Pathol. 1987 May;87(5):569–575. doi: 10.1093/ajcp/87.5.569. [DOI] [PubMed] [Google Scholar]
- Weller A. H., Simpson K., Herzum M., Van Houten N., Huber S. A. Coxsackievirus-B3-induced myocarditis: virus receptor antibodies modulate myocarditis. J Immunol. 1989 Sep 15;143(6):1843–1850. [PubMed] [Google Scholar]




