Abstract
To examine the interaction of the poliovirus receptor (PVR) with virus and the role of the PVR in virus entry, the PVR was expressed in insect cells. Poliovirus bound to insect cells infected with a recombinant baculovirus (AcPVR) carrying cDNA encoding the PVR. Antibodies raised against PVR expressed in bacteria immunoprecipitated a 67-kilodalton polypeptide from cytoplasmic extracts of AcPVR-infected cells. Treatment of AcPVR-infected cells with tunicamycin revealed that the PVR is a glycoprotein containing N-glycosidic linkages and that carbohydrate accounts for nearly 50% of its molecular weight as estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. When PVR was solubilized from AcPVR-infected insect cells and incubated with poliovirus, viral infectivity was neutralized. Sedimentation analysis revealed that irreversibly altered 135S particles were formed after incubation of poliovirus at 37 degrees C with solubilized extracts of AcPVR-infected insect cells. These results demonstrate that poliovirus eclipse may result from interaction with the cell receptor at neutral pH in the absence of membranes and suggest that soluble receptors may be effective antiviral agents against picornaviruses.
Full text
PDF
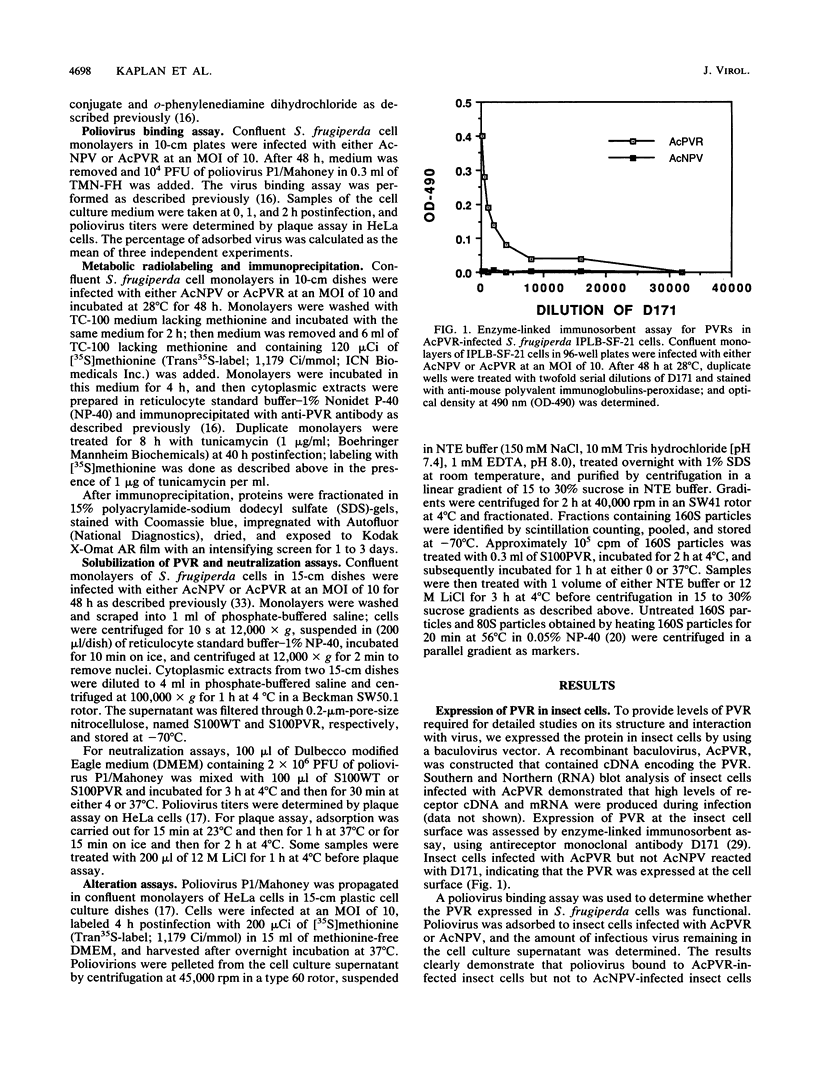
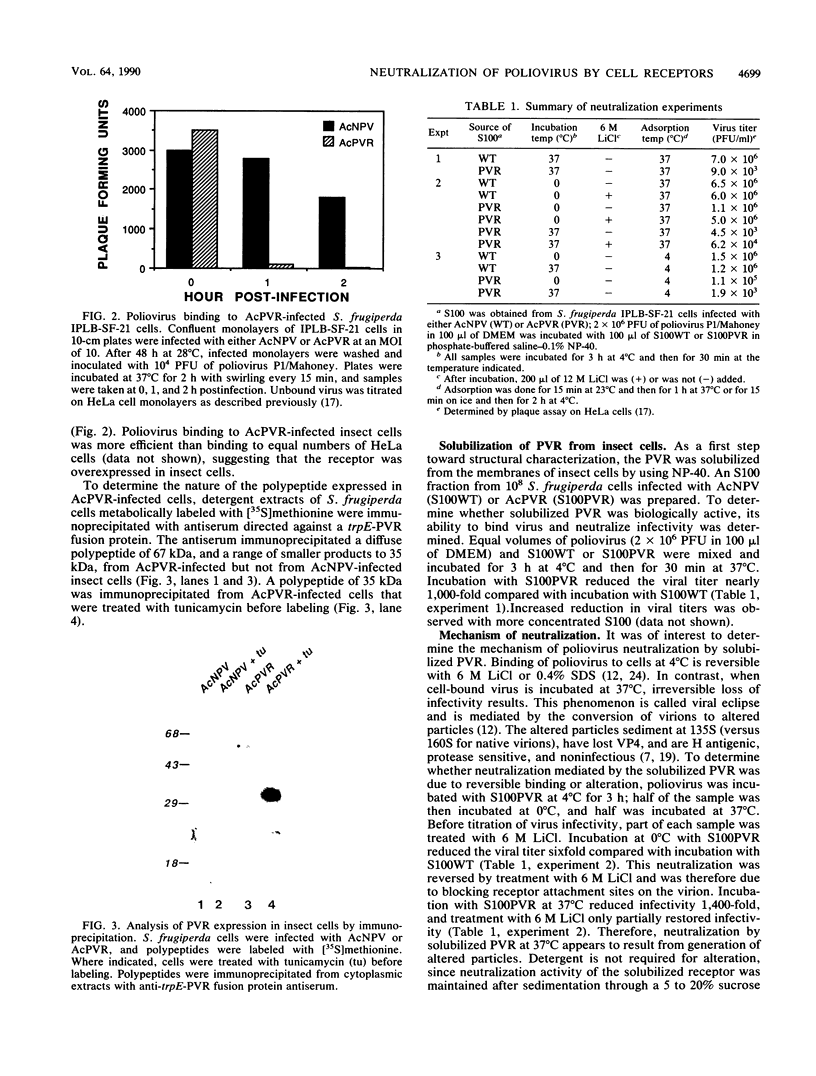
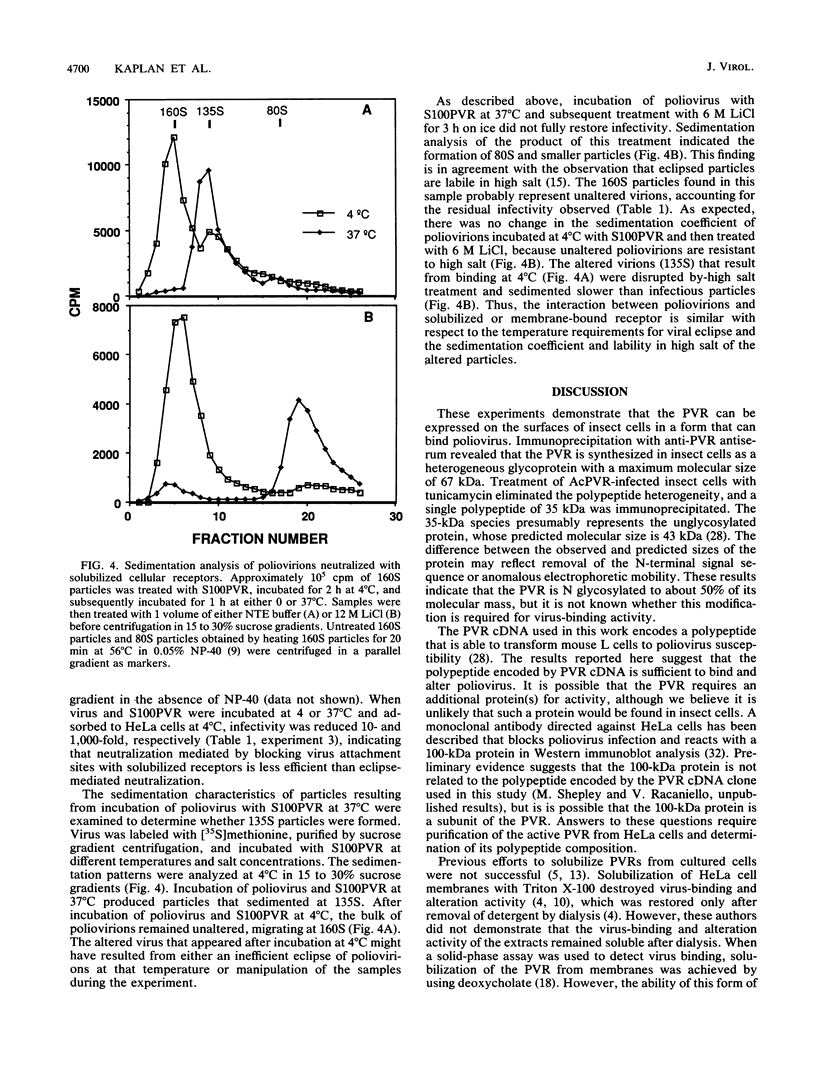


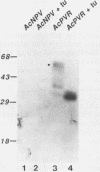
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger E. A., Fuerst T. R., Moss B. A soluble recombinant polypeptide comprising the amino-terminal half of the extracellular region of the CD4 molecule contains an active binding site for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2357–2361. doi: 10.1073/pnas.85.7.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., McSharry J. J., Lawrence G. W. Effect of arildone on modifications of poliovirus in vitro. Virology. 1980 Aug;105(1):86–93. doi: 10.1016/0042-6822(80)90158-0. [DOI] [PubMed] [Google Scholar]
- De Sena J., Heifner W. R., Stolov N. S. Studies on the in vitro uncoating of poliovirus. IV. Characteristics of solubilized membrane-modifying and -stabilizing factors. Virology. 1983 Jul 30;128(2):354–365. doi: 10.1016/0042-6822(83)90262-3. [DOI] [PubMed] [Google Scholar]
- De Sena J., Mandel B. Studies on the in vitro uncoating of poliovirus. I. Characterization of the modifying factor and the modifying reaction. Virology. 1976 Apr;70(2):470–483. doi: 10.1016/0042-6822(76)90288-9. [DOI] [PubMed] [Google Scholar]
- Deen K. C., McDougal J. S., Inacker R., Folena-Wasserman G., Arthos J., Rosenberg J., Maddon P. J., Axel R., Sweet R. W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988 Jan 7;331(6151):82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- Dunnebacke T. H., Levinthal J. D., Williams R. C. Entry and release of poliovirus as observed by electron microscopy of cultured cells. J Virol. 1969 Oct;4(4):505–513. doi: 10.1128/jvi.4.4.505-513.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaert L., Vrijsen R., Boeyé A. Eclipse products of poliovirus after cold-synchronized infection of HeLa cells. Virology. 1989 Jul;171(1):76–82. doi: 10.1016/0042-6822(89)90512-6. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Bertonis J. M., Meier W., Johnson V. A., Costopoulos D. S., Liu T., Tizard R., Walker B. D., Hirsch M. S., Schooley R. T. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988 Jan 7;331(6151):76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- Fricks C. E., Hogle J. M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990 May;64(5):1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromeier M., Wetz K. Kinetics of poliovirus uncoating in HeLa cells in a nonacidic environment. J Virol. 1990 Aug;64(8):3590–3597. doi: 10.1128/jvi.64.8.3590-3597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman N., Baltimore D. A plasma membrane component able to bind and alter virions of poliovirus type 1: studies on cell-free alteration using a simplified assay. Virology. 1977 Oct 1;82(1):25–36. doi: 10.1016/0042-6822(77)90029-0. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., HOYER B. H. Early stages of enterovirus infection. Cold Spring Harb Symp Quant Biol. 1962;27:101–112. doi: 10.1101/sqb.1962.027.001.013. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The location and nature of enterovirus receptors in susceptible cells. J Exp Med. 1961 Aug 1;114:161–171. doi: 10.1084/jem.114.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey R. E., Richardson N. E., Kowalski M., Brown N. R., Chang H. C., Siliciano R. F., Dorfman T., Walker B., Sodroski J., Reinherz E. L. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988 Jan 7;331(6151):78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Levy A., Racaniello V. R. Isolation and characterization of HeLa cell lines blocked at different steps in the poliovirus life cycle. J Virol. 1989 Jan;63(1):43–51. doi: 10.1128/jvi.63.1.43-51.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Racaniello V. R. Construction and characterization of poliovirus subgenomic replicons. J Virol. 1988 May;62(5):1687–1696. doi: 10.1128/jvi.62.5.1687-1696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krah D. L., Crowell R. L. A solid-phase assay of solubilized HeLa cell membrane receptors for binding group B coxsackieviruses and polioviruses. Virology. 1982 Apr 15;118(1):148–156. doi: 10.1016/0042-6822(82)90328-2. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Gosser L. B., Kauer J. C. Early alteration of poliovirus in infected cells and its specific inhibition. J Gen Virol. 1975 Jun;27(3):329–342. doi: 10.1099/0022-1317-27-3-329. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Gosser L. B., Shimshick E. J. Interaction of liposomes with subviral particles of poliovirus type 2 and rhinovirus type 2. J Virol. 1976 Aug;19(2):746–749. doi: 10.1128/jvi.19.2.746-749.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early interaction between animal viruses and cells. Monogr Virol. 1974;9:1–148. [PubMed] [Google Scholar]
- MANDEL B. The use of sodium dodecyl sulfate in studies on the interaction of poliovirus and HeLa cells. Virology. 1962 Jun;17:288–294. doi: 10.1016/0042-6822(62)90119-8. [DOI] [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S., Sandvig K. Mechanism of entry into the cytosol of poliovirus type 1: requirement for low pH. J Cell Biol. 1984 Apr;98(4):1194–1200. doi: 10.1083/jcb.98.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S., Sandvig K. Requirements for entry of poliovirus RNA into cells at low pH. EMBO J. 1984 Sep;3(9):1945–1950. doi: 10.1002/j.1460-2075.1984.tb02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Staunton D. E., Springer T. A., Stratowa C., Sommergruber W., Merluzzi V. J. A soluble form of intercellular adhesion molecule-1 inhibits rhinovirus infection. Nature. 1990 Mar 1;344(6261):70–72. doi: 10.1038/344070a0. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Caliguiri L. A., Eggers H. J. Inhibition of uncoating of poliovirus by arildone, a new antiviral drug. Virology. 1979 Sep;97(2):307–315. doi: 10.1016/0042-6822(79)90342-8. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Nobis P., Zibirre R., Meyer G., Kühne J., Warnecke G., Koch G. Production of a monoclonal antibody against an epitope on HeLa cells that is the functional poliovirus binding site. J Gen Virol. 1985 Dec;66(Pt 12):2563–2569. doi: 10.1099/0022-1317-66-12-2563. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R. Cell receptors for picornaviruses. Curr Top Microbiol Immunol. 1990;161:1–22. doi: 10.1007/978-3-642-75602-3_1. [DOI] [PubMed] [Google Scholar]
- Shepley M. P., Sherry B., Weiner H. L. Monoclonal antibody identification of a 100-kDa membrane protein in HeLa cells and human spinal cord involved in poliovirus attachment. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7743–7747. doi: 10.1073/pnas.85.20.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traunecker A., Lüke W., Karjalainen K. Soluble CD4 molecules neutralize human immunodeficiency virus type 1. Nature. 1988 Jan 7;331(6151):84–86. doi: 10.1038/331084a0. [DOI] [PubMed] [Google Scholar]
- Willingmann P., Barnert H., Zeichhardt H., Habermehl K. O. Recovery of structurally intact and infectious poliovirus type 1 from HeLa cells during receptor-mediated endocytosis. Virology. 1989 Feb;168(2):417–420. doi: 10.1016/0042-6822(89)90286-9. [DOI] [PubMed] [Google Scholar]
- Zeichhardt H., Wetz K., Willingmann P., Habermehl K. O. Entry of poliovirus type 1 and Mouse Elberfeld (ME) virus into HEp-2 cells: receptor-mediated endocytosis and endosomal or lysosomal uncoating. J Gen Virol. 1985 Mar;66(Pt 3):483–492. doi: 10.1099/0022-1317-66-3-483. [DOI] [PubMed] [Google Scholar]