Abstract
Medullary serotonergic (5-HT) neurons are implicated in central chemoreception and 5-HT abnormalities are present in many cases of the sudden infant death syndrome (SIDS). Mice with a targeted disruption of the serotonin transporter (5-HTT) develop in the presence of excess 5-HT in brain extracellular fluid (ECF). As adults they exhibit reduced 5-HT neuron activity and 5-HT1A receptor binding with varying changes in postsynaptic 5-HT receptor function. They exhibit behavioural phenotypes (anxiety, reduced aggression) but little is known about their control of breathing. We show that conscious adult male and female 5-HTT knockout mice breathing air at room temperature have a higher resting 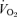 , breathing frequency and
, breathing frequency and 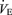 but a normal body temperature and
but a normal body temperature and 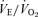 ratio (the ventilatory equivalent) compared to wild-type (WT) controls. In hypercapnia, there is a reduced ventilatory response (expressed as the
ratio (the ventilatory equivalent) compared to wild-type (WT) controls. In hypercapnia, there is a reduced ventilatory response (expressed as the 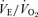 ratio) that is much more prominent in males (−68%) than females (−22%). In hypoxia, both males and females exhibit a higher
ratio) that is much more prominent in males (−68%) than females (−22%). In hypoxia, both males and females exhibit a higher 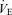 ,
, 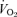 and body temperature but their
and body temperature but their 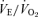 ratio is normal. We conclude that 5-HTT knockout mice have a diminished function of the medullary 5-HT system, which is manifest most remarkably in a substantial loss of CO2 sensitivity predominantly in males. This finding supports the importance of medullary 5-HT neurons in central chemoreception. Females either rely less on 5-HT neurons in chemoreception or adapt more readily to the loss of 5-HT function. This genetic model allows examination of the role of excess 5-HT in ECF in the development of the control of breathing and central chemoreception, which may be pertinent to SIDS.
ratio is normal. We conclude that 5-HTT knockout mice have a diminished function of the medullary 5-HT system, which is manifest most remarkably in a substantial loss of CO2 sensitivity predominantly in males. This finding supports the importance of medullary 5-HT neurons in central chemoreception. Females either rely less on 5-HT neurons in chemoreception or adapt more readily to the loss of 5-HT function. This genetic model allows examination of the role of excess 5-HT in ECF in the development of the control of breathing and central chemoreception, which may be pertinent to SIDS.
Central chemoreception, the process by which the brainstem detects changes in pH or 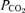 and increases breathing, is present at many locations and involves more than one type of neuron (Nattie & Li, 2001, 2006; Feldman et al. 2003; Hodges et al. 2004, 2008; Nattie et al. 2004; Guyenet et al. 2005; Richerson et al. 2005; Taylor et al. 2005; Li et al. 2006; Mulkey et al. 2007). Medullary serotonergic (5-HT) neurons have been proposed as important participants in central chemoreception as CO2 detectors (Richerson et al. 2005) and as modulators of other detector neurons (Li et al. 2006; Mulkey et al. 2007; Hodges et al. 2008). It is not clear which type of neuron or which chemoreceptor location is of greatest importance in normal physiology (Feldman et al. 2003; Guyenet et al. 2005; Nattie & Li, 2006; Mulkey et al. 2007).
and increases breathing, is present at many locations and involves more than one type of neuron (Nattie & Li, 2001, 2006; Feldman et al. 2003; Hodges et al. 2004, 2008; Nattie et al. 2004; Guyenet et al. 2005; Richerson et al. 2005; Taylor et al. 2005; Li et al. 2006; Mulkey et al. 2007). Medullary serotonergic (5-HT) neurons have been proposed as important participants in central chemoreception as CO2 detectors (Richerson et al. 2005) and as modulators of other detector neurons (Li et al. 2006; Mulkey et al. 2007; Hodges et al. 2008). It is not clear which type of neuron or which chemoreceptor location is of greatest importance in normal physiology (Feldman et al. 2003; Guyenet et al. 2005; Nattie & Li, 2006; Mulkey et al. 2007).
Striking abnormalities in the medullary 5-HT system have been reported in cases of sudden infant death syndrome (SIDS) (Paterson et al. 2006). Many SIDS cases have an increased number of 5-HT neurons and exhibit decreased binding of the 5-HT1A autoreceptor and serotonin transporter (5-HTT) (Paterson et al. 2006). How such abnormalities translate into a physiological mechanism for sudden and unexpected death in an apparently healthy infant remains a mystery (Paterson et al. 2006; Thach, 2008).
We hypothesize that medullary 5-HT neurons are of special importance in central chemoreception, in the development of the control of breathing and in the pathogenesis of SIDS. As one experimental model in the examination of this hypothesis we employed mice with a targeted disruption of the serotonin transporter (5-HTT) (Holmes et al. 2003). These mice develop in the presence of excess 5-HT in brain extracellular fluid (ECF). As adults they exhibit increased 5-HT in ECF (Mathews et al. 2004; Kim et al. 2005), decreased tissue 5-HT (Kim et al. 2005), increased 5-HT synthesis (Kim et al. 2005), reduced 5-HT neuron activity (Gobbi et al. 2001), reduced 5-HT1A receptor binding (Li et al. 2000; Bouali et al. 2003) and have varying changes in postsynaptic 5-HT receptor function (Qu et al. 2005). This neurochemical phenotype includes two of the three abnormalities described in the brainstem of the SIDS cases, decreased 5-HT1A and 5-HTT binding. The 5-HTT knockout mice exhibit behavioural phenotypes: increased anxiety-like behaviours, reduced aggression, and exaggerated stress responses (Holmes et al. 2003; Adamec et al. 2006) but little is known about their physiology. There are known sex differences in the 5-HTT knockout. Female as compared to male adult 5-HTT knockout mice appear to have a greater desensitization of dorsal raphe 5-HT neurons (Bouali et al. 2003) and a greater increase in brain 5-HT synthesis (Kim et al. 2005) and there are sex differences in the incidence of SIDS (60 : 40; male predominance; see Centers for Disease Control and Prevention, 2008). In this study we examine breathing, body temperature and oxygen consumption in air and in response to increased CO2 or decreased O2 in unanaesthetized adult male and female 5-HTT knockout and wild-type mice. Our hypothesis is that with long-term excess of 5-HT in ECF these 5-HTT knockout mice will have a brainstem 5-HT system that is ‘turned down’, i.e. is less responsive. We expect to see a decreased CO2 response of a degree commensurate with the importance of 5-HT neurons in central chemoreception.
Methods
Ethical approval
All experimentation procedures and protocols were within the guidelines of the National Institutes of Health for animal use and care and were approved by the Dartmouth College Institutional Animal Use and Care Committee. The mice were never anaesthetized nor were they killed. The 5-HTT knockout and the WT control mice both of the C57BL/6 strain were acquired from Taconic Farm and tested at age 5–6 months. We verified their genotype before the experiments. In brief, the tail samples were digested and subjected to polymerase chain reaction (PCR) with three primers for 5-HTT (5-HTT-A primer: 5′-TCT ATG GGA AGG CTG ACA GGT-3′; 5-HTT-B primer: 5′-TTG CTG ACT GGA GTA CAG GCT A-3′; and NEO primer: 5′-TCG ACG TTG TCA CTG AAG CGG-3′). The PCR product was analysed by electrophoresis. The 5-HTT wild-type allele is identified at 1.4 kb, and the 5-HTT knockout allele is identified at 1 kb. The mice were housed in a room in the Animal Resource Center with a light, rest period from midnight to noon and a dark, active period from noon to midnight. Food and water were available ad libitum. All the experiments were performed between 8 am and 3 pm. Ventilation 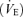 , tidal volume (VT), breathing frequency (f), and oxygen consumption
, tidal volume (VT), breathing frequency (f), and oxygen consumption 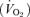 were measured in non-instrumented WT and 5-HTT knockout mice of both sexes using whole-body plethysmography during wakefulness while breathing room air, 5% CO2 or 10% O2. We studied a total of 24 mice; five and six WT males and females, respectively, and seven and six 5-HTT knockout males and females, respectively. The rectal temperature was measured before and after each experiment using a small thermistor probe. The volume of the plethysmograph was ∼214 ml (5.5 mm diameter, 9 mm long cylinder). The inflow gas for the plethysmograph chamber was humidified and controlled by a flowmeter at a minimum of 0.4 l min−1. The outflow was matched to the inflow via a flowmeter connected to a vacuum system. Approximately 100 ml min−1 of outflow gas served O2 and CO2 analysers (Applied Electrochemistry). We measured chamber pressure by transducer and calibrated the plethysmograph with multiple 0.1 ml injections. The chamber temperature was measured by a thermometer continuously. After the mouse was acclimatized to the plethysmograph chamber (usually ∼30 min), data were obtained over 20–30 min of breathing air and during the last 5 min of a 15–20 min period of exposure to 5% CO2 (5% CO2, 21% O2, remainder N2) or 10% O2 (10% O2, remainder N2). These two responses were tested on separate days. Tidal volume (VT) was calculated using plethysmograph temperature at that time and mouse temperature measured closest to that time (Li et al. 2006), and breathing frequency (f) per breath to estimate ventilation
were measured in non-instrumented WT and 5-HTT knockout mice of both sexes using whole-body plethysmography during wakefulness while breathing room air, 5% CO2 or 10% O2. We studied a total of 24 mice; five and six WT males and females, respectively, and seven and six 5-HTT knockout males and females, respectively. The rectal temperature was measured before and after each experiment using a small thermistor probe. The volume of the plethysmograph was ∼214 ml (5.5 mm diameter, 9 mm long cylinder). The inflow gas for the plethysmograph chamber was humidified and controlled by a flowmeter at a minimum of 0.4 l min−1. The outflow was matched to the inflow via a flowmeter connected to a vacuum system. Approximately 100 ml min−1 of outflow gas served O2 and CO2 analysers (Applied Electrochemistry). We measured chamber pressure by transducer and calibrated the plethysmograph with multiple 0.1 ml injections. The chamber temperature was measured by a thermometer continuously. After the mouse was acclimatized to the plethysmograph chamber (usually ∼30 min), data were obtained over 20–30 min of breathing air and during the last 5 min of a 15–20 min period of exposure to 5% CO2 (5% CO2, 21% O2, remainder N2) or 10% O2 (10% O2, remainder N2). These two responses were tested on separate days. Tidal volume (VT) was calculated using plethysmograph temperature at that time and mouse temperature measured closest to that time (Li et al. 2006), and breathing frequency (f) per breath to estimate ventilation 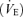 per breath.
per breath.
Statistics
All data analysis was performed within sexes to avoid complications of normalization due to sex-related differences in age and body weight. Baseline age, body weight, body temperature, 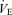 and
and 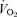 values were compared within each sex by t test. We analysed the responses to hypercapnia or hypoxia using a repeated measures one-way ANOVA with the ventilatory variable, body temperature or
values were compared within each sex by t test. We analysed the responses to hypercapnia or hypoxia using a repeated measures one-way ANOVA with the ventilatory variable, body temperature or 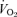 as the repeated measure and the treatment being wild-type versus 5-HTT knockout. Post hoc Tukey's test was applied as indicated by a significant interactive term in the ANOVA. Data for breathing room air at rest are pooled from data before the CO2 and before the hypoxic tests.
as the repeated measure and the treatment being wild-type versus 5-HTT knockout. Post hoc Tukey's test was applied as indicated by a significant interactive term in the ANOVA. Data for breathing room air at rest are pooled from data before the CO2 and before the hypoxic tests.
Results
Male WT and 5-HTT knockouts were the same age and body weight on average at the time of these studies. Female mice were older but of lower body weight than males but there was no difference between female WT and 5-HTT knockouts (Table 1). While breathing air (Table 1), male 5-HTT knockout mice had a faster breathing frequency (P < 0.01) and a greater 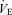 (P < 0.01) compared to male WT. Resting
(P < 0.01) compared to male WT. Resting 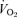 was also greater (P < 0.05) such that the ventilatory equivalent
was also greater (P < 0.05) such that the ventilatory equivalent 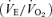 was not affected by the 5-HTT knockout. While breathing air (Table 1), female 5-HTT knockout mice had a faster breathing frequency (P < 0.01) and a greater
was not affected by the 5-HTT knockout. While breathing air (Table 1), female 5-HTT knockout mice had a faster breathing frequency (P < 0.01) and a greater 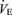 compared to female WT although this did not reach statistical significance. Resting
compared to female WT although this did not reach statistical significance. Resting 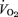 was also greater (P < 0.05) and the ventilatory equivalent
was also greater (P < 0.05) and the ventilatory equivalent 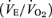 was not affected by the 5-HTT knockout. Body temperature did not differ between WT and 5-HTT knockout mice of either sex at room temperature during air breathing (Table 1).
was not affected by the 5-HTT knockout. Body temperature did not differ between WT and 5-HTT knockout mice of either sex at room temperature during air breathing (Table 1).
Table 1.
Age, body weight, baseline ventilation and oxygen consumption of wild-type control and 5-HTT knockout mice
| Age (days) | Body weight (g) | f (min−1) | VT (ml g−1) |
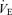 (ml min−1 g−1) (ml min−1 g−1) |
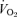 (ml min−1 g−1) (ml min−1 g−1) |
Tbody (°C) | ||
|---|---|---|---|---|---|---|---|---|
| Male WT (n= 5) | 163 ± 12 | 31 ± 2 | 205 ± 5 | 0.012 ± 0.001 | 2.31 ± 0.09 | 0.060 ± 0.002 | 36.6 ± 0.2 | 39 ± 2 |
| Male KO (n= 7) | 165 ± 7 | 29 ± 1 | 254 ± 9 | 0.011 ± 0.0002 | 2.90 ± 0.11 | 0.080 ± 0.002 | 37.0 ± 0.2 | 37 ± 2 |
| n.s. | n.s. | * | n.s. | * | + | n.s. | n.s. | |
| Female WT (n= 6) | 181 ± 10 | 24 ± 1 | 192 ± 9 | 0.015 ± 0.001 | 2.83 ± 0.21 | 0.078 ± 0.003 | 36.5 ± 0.2 | 37 ± 2 |
| Female KO (n= 6) | 168 ± 8 | 25 ± 1 | 235 ± 6 | 0.014 ± 0.001 | 3.35 ± 0.18 | 0.093 ± 0.005 | 36.8 ± 0.2 | 36 ± 2 |
| n.s. | n.s. | * | n.s. | n.s. | + | n.s. | n.s. |
Values are means ±s.e.m.
P < 0.01; +P < 0.05; t test applied within each sex. f, breathing frequency; VT, tidal volume; 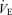 , ventilation;
, ventilation; 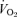 , oxygen consumption; Tbody, body temperature;
, oxygen consumption; Tbody, body temperature; 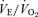 , ventilatory equivalent.
, ventilatory equivalent.
We evaluated the ventilatory response to increased CO2 first by normalizing the data to body weight. Figure 1 shows that the 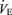 response to CO2 was markedly reduced in male 5-HTT knockout mice compared to wild-type (P < 0.001, treatment effect and interactive term of one-way repeated measures ANOVA; P < 0.01, post hoc comparison at 5% CO2), an effect entirely due to a reduced VT response (P < 0.001, treatment effect and interactive term of one-way repeated measures ANOVA; P < 0.01, post hoc comparison at 5% CO2).
response to CO2 was markedly reduced in male 5-HTT knockout mice compared to wild-type (P < 0.001, treatment effect and interactive term of one-way repeated measures ANOVA; P < 0.01, post hoc comparison at 5% CO2), an effect entirely due to a reduced VT response (P < 0.001, treatment effect and interactive term of one-way repeated measures ANOVA; P < 0.01, post hoc comparison at 5% CO2).  and body temperature did not change during exposure to 5% CO2 in any group (data not shown). In females the
and body temperature did not change during exposure to 5% CO2 in any group (data not shown). In females the 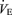 response to CO2 was also reduced (P < 0.001, treatment effect and P < 0.03 interactive term of one-way repeated measures ANOVA). If we define the CO2 response as the percentage increase in
response to CO2 was also reduced (P < 0.001, treatment effect and P < 0.03 interactive term of one-way repeated measures ANOVA). If we define the CO2 response as the percentage increase in 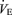 from the air breathing value, the WT males have a 183% increase compared to an 81% increase in the 5-HTT knockout mice. The male knockouts have a 56% reduction in the CO2 response so defined; the females a 34% reduction. If we define the CO2 response as the change in
from the air breathing value, the WT males have a 183% increase compared to an 81% increase in the 5-HTT knockout mice. The male knockouts have a 56% reduction in the CO2 response so defined; the females a 34% reduction. If we define the CO2 response as the change in 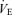 from air to 5% CO2 breathing, in WT male mice Δ is 4.56 ml min−1 g−1 while in 5-HTT knockouts Δ is 2.3 ml min−1 g−1, a reduction of 48% in this measure of the CO2 response; the females have a 21% reduction.
from air to 5% CO2 breathing, in WT male mice Δ is 4.56 ml min−1 g−1 while in 5-HTT knockouts Δ is 2.3 ml min−1 g−1, a reduction of 48% in this measure of the CO2 response; the females have a 21% reduction.
Figure 1.
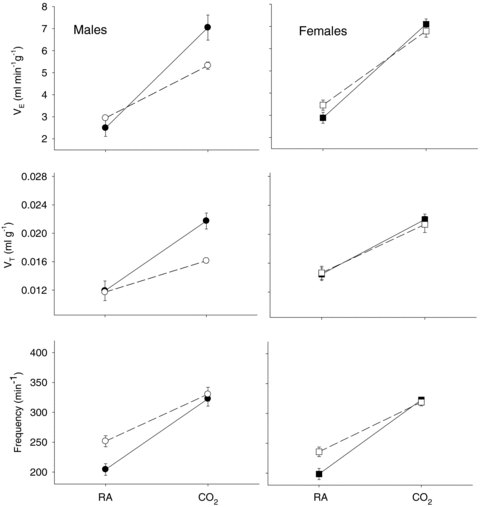
The CO2 responses in unanaesthetized, unrestrained male (left) and female (right) wild-type (•; n = 5, 6) and 5-HTT knockout mice (○; n = 7, 6) are shown as the ventilation 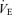 , tidal volume VT, and breathing frequency, while breathing room air (RA) or 5% CO2 in air (CO2) during wakefulness. The symbols show the mean values and error bars show s.e.m. At 5% CO2,
, tidal volume VT, and breathing frequency, while breathing room air (RA) or 5% CO2 in air (CO2) during wakefulness. The symbols show the mean values and error bars show s.e.m. At 5% CO2, 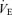 is significantly less in the 5-HTT versus wild-type in males as is VT (P < 0.01, post hoc test with significant interactive term of ANOVA). Breathing frequency is significantly greater during air breathing in the 5-HTT mice of both sexes (P < 0.01, post hoc test with significant interactive term of ANOVA).
is significantly less in the 5-HTT versus wild-type in males as is VT (P < 0.01, post hoc test with significant interactive term of ANOVA). Breathing frequency is significantly greater during air breathing in the 5-HTT mice of both sexes (P < 0.01, post hoc test with significant interactive term of ANOVA).
To take into consideration the large difference in resting 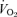 between the knockout and WT mice, we also normalized the ventilatory output as
between the knockout and WT mice, we also normalized the ventilatory output as 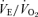 . The CO2 response was again markedly reduced in male 5-HTT knockout mice compared to male wild-type (Fig. 2) (P < 0.001, treatment effect and interactive term of one-way repeated measures ANOVA). In female mice the CO2 response was also significantly reduced in the 5-HTT knockout mice compared to male WT (Fig. 2) (P < 0.05, treatment effect but no interactive term of one-way repeated measures ANOVA) but the degree of the reduction was less. In the male mice, the reduction in the
. The CO2 response was again markedly reduced in male 5-HTT knockout mice compared to male wild-type (Fig. 2) (P < 0.001, treatment effect and interactive term of one-way repeated measures ANOVA). In female mice the CO2 response was also significantly reduced in the 5-HTT knockout mice compared to male WT (Fig. 2) (P < 0.05, treatment effect but no interactive term of one-way repeated measures ANOVA) but the degree of the reduction was less. In the male mice, the reduction in the 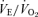 ratio while breathing 5% CO2 was 50%. If we define the CO2 response as the percentage increase in
ratio while breathing 5% CO2 was 50%. If we define the CO2 response as the percentage increase in 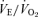 from the air breathing value, the WT males have a 252% increase compared to an 88% increase in the 5-HTT knockout mice. The male knockouts have a 65% reduction in the CO2 response so defined; the females have a 15% reduction. If we define the CO2 response as the change in the
from the air breathing value, the WT males have a 252% increase compared to an 88% increase in the 5-HTT knockout mice. The male knockouts have a 65% reduction in the CO2 response so defined; the females have a 15% reduction. If we define the CO2 response as the change in the 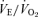 ratio from air to 5% CO2 breathing, in WT male mice Δ is 96 while in the 5-HTT knockouts Δ is 31, a reduction of 68% in this measure of the CO2 response; the females have a 22% reduction.
ratio from air to 5% CO2 breathing, in WT male mice Δ is 96 while in the 5-HTT knockouts Δ is 31, a reduction of 68% in this measure of the CO2 response; the females have a 22% reduction.
Figure 2.
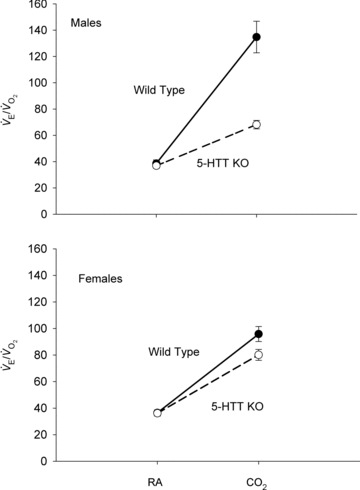
The CO2 responses in unanaesthetized, unrestrained male (top) and female (bottom) wild-type (•; n = 5, 6) and 5-HTT knockout mice (○; n = 7, 6) are shown as the ventilatory equivalent, 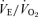 , while breathing room air (RA) or 5% CO2 in air (CO2) during wakefulness. The symbols show the mean values and error bars show s.e.m. At 5% CO2,
, while breathing room air (RA) or 5% CO2 in air (CO2) during wakefulness. The symbols show the mean values and error bars show s.e.m. At 5% CO2, 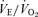 is significantly less in the 5-HTT versus wild-type in both sexes; P < 0.01, post hoc test with significant interactive term of ANOVA.
is significantly less in the 5-HTT versus wild-type in both sexes; P < 0.01, post hoc test with significant interactive term of ANOVA.
The response to breathing 10% O2 in small rodents includes changes in body temperature, 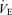 ,
, 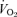 and
and 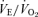 , which are shown in Figs 3 (males) and 4 (females). The male 5-HTT knockout mice compared to the wild-type (Fig. 3) have a significantly greater
, which are shown in Figs 3 (males) and 4 (females). The male 5-HTT knockout mice compared to the wild-type (Fig. 3) have a significantly greater 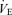 (P < 0.001 for treatment effect; no significant interaction) and
(P < 0.001 for treatment effect; no significant interaction) and 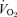 (P < 0.001 for treatment effect; no significant interaction) such that the response of
(P < 0.001 for treatment effect; no significant interaction) such that the response of 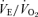 is the same as in controls. The female 5-HTT knockout mice compared to the wild-type (Fig. 4) have a significantly greater
is the same as in controls. The female 5-HTT knockout mice compared to the wild-type (Fig. 4) have a significantly greater 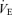 (P < 0.001 for treatment effect; P < 0.02 for the interactive effect) and
(P < 0.001 for treatment effect; P < 0.02 for the interactive effect) and  (P < 0.001 for treatment effect; no significant interaction) such that the response of
(P < 0.001 for treatment effect; no significant interaction) such that the response of 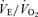 is the same as in controls. In both sexes the 5-HTT knockout mice have a smaller drop in body temperature as a result of hypoxic exposure compared to the wild-type controls (P < 0.05 treatment effect; P < 0.05 for the interactive effect).
is the same as in controls. In both sexes the 5-HTT knockout mice have a smaller drop in body temperature as a result of hypoxic exposure compared to the wild-type controls (P < 0.05 treatment effect; P < 0.05 for the interactive effect).
Figure 3.
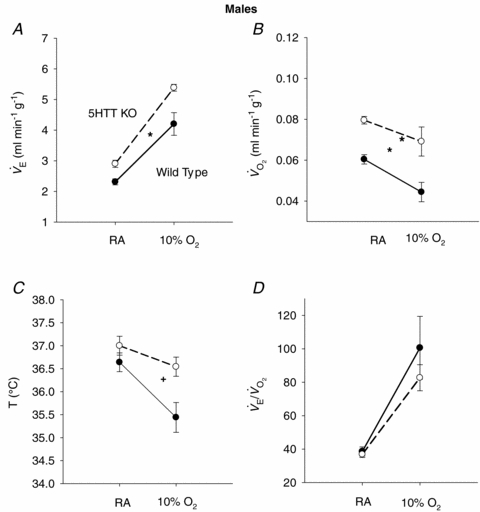
The responses of 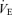 (A),
(A), 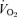 (B), body temperature (C), and
(B), body temperature (C), and 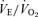 (D) in unanaesthetized, unrestrained male wild-type (•; n = 5) and 5-HTT knockout mice (○; n = 7) are shown while breathing room air (RA) or 10% O2 in nitrogen during wakefulness. The symbols show the mean values and error bars show s.e.m.*P < 0.001; +P < 0.05, for main treatment and interactive effect.
(D) in unanaesthetized, unrestrained male wild-type (•; n = 5) and 5-HTT knockout mice (○; n = 7) are shown while breathing room air (RA) or 10% O2 in nitrogen during wakefulness. The symbols show the mean values and error bars show s.e.m.*P < 0.001; +P < 0.05, for main treatment and interactive effect.
Figure 4.
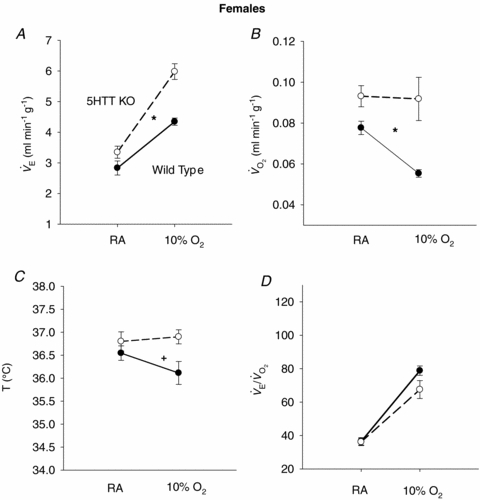
The responses of 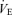 (A),
(A), 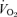 (B), body temperature (C), and
(B), body temperature (C), and 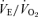 (D) in unanaesthetized, unrestrained female wild-type (•; n = 6) and 5-HTT knockout mice (○; n = 6) are shown while breathing room air (RA) or 10% O2 in nitrogen during wakefulness. The symbols show the mean values and error bars show s.e.m.*P < 0.001; +P < 0.05, for main treatment and interactive effect.
(D) in unanaesthetized, unrestrained female wild-type (•; n = 6) and 5-HTT knockout mice (○; n = 6) are shown while breathing room air (RA) or 10% O2 in nitrogen during wakefulness. The symbols show the mean values and error bars show s.e.m.*P < 0.001; +P < 0.05, for main treatment and interactive effect.
Discussion
Our hypothesis was supported by the major finding of the study, a substantial decrease in the CO2 response in the 5-HTT knockout mice compared to WT controls. Our findings complement the recent observation that mice with total absence of brainstem 5-HT neurons due to conditional knockout of the transcription factor Lmx1b, which is necessary for their final determination, also have a markedly reduced CO2 response (Hodges et al. 2008). However, in our experiments there is excess 5-HT in ECF while in the Lmx1b conditional knockout there is an absence of 5-HT neurons. These findings indicate that 5-HT neurons play an important role in central chemoreception but do not show whether this 5-HT effect is via a direct chemosensing property of 5-HT neurons (Richerson et al. 2005) or via an indirect effect by which medullary 5-HT neuronal output can alter the chemosensitivity of other medullary neurons, e.g. in the retrotrapezoid nucleus (Li et al. 2006; Mulkey et al. 2007). As 5-HT participates in carotid body function (Jacono et al. 2005) the 5-HTT knockout could, in addition, have altered the peripheral chemoreceptor response to CO2. The absence of an effect on the response of the 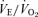 ratio to hypoxia provides some evidence that carotid body function is relatively normal in the 5-HTT knockout.
ratio to hypoxia provides some evidence that carotid body function is relatively normal in the 5-HTT knockout.
The male predominance of the 5-HTT knockout effects on the CO2 response is unexpected. Other studies of central chemoreception involving 5-HT neurons have predominantly been in males (Nattie & Li, 2001; Nattie et al. 2004; Taylor et al. 2004, 2005; Li et al. 2006; Hodges et al. 2008). One study that did examine both sexes after lesions of 5-HT neurons in newborn piglets did find a reduced CO2 response only in males and only in NREM sleep (Penatti et al. 2006). These data show that the role of 5-HT neurons in chemoreception varies with sex and raise the provocative issue of whether chemoreception per se involves different central chemosensitive neurons or regions in males and females.
One possible explanation for the lesser effect of the 5-HTT knockout on the CO2 response in females is a sex difference in adaptability or plasticity. Behan et al. (2002) found that long-term facilitation (LTF), a form of respiratory plasticity in which repeated exposures to hypoxic stimulation result in a lasting increase in respiratory output, was greater in older (13 months) than in younger females (3–4 months) while in males it was reversed. In our mice, the relative absence of any reduction in CO2 sensitivity in adult females could represent more effective adaptation, perhaps analogous to LTF.
Other studies of the 5-HTT knockout mouse have uncovered sex differences in phenotype. Administration of the 5-HT1A agonist 8-OH-DPAT to small rodents causes hypothermia due to inhibition of brainstem 5-HT neurons that (a) stimulate brown fat metabolism to generate heat, and (b) vasoconstrict blood vessels to conserve heat (Morrison, 2004; Ootsuka & Blessing, 2006). The 5-HT neurons in 5-HTT knockout mice have been chronically exposed to excess 5-HT and their 5-HT1A receptors are down-regulated by adulthood; the neurons exhibit less inhibition of firing rates and the mice less hypothermia when exposed to 8-OH-DPAT (Bouali et al. 2003) and this down-regulation is greater in females than in males (Li et al. 2000; Bouali et al. 2003). Perhaps in males the 5-HT1A autoreceptors are less affected by the excess 5-HT in ECF, a conclusion supported by the observation that castration in male 5-HTT knockout mice resulted in female-like down-regulation of 5HT1A function (Bouali et al. 2003). Or males could be exposed to smaller increases of 5-HT in ECF as the increased brain 5-HT synthesis observed in 5-HTT knockout mice is greater in females than males (Kim et al. 2005). Both of these explanations presume that 5-HT neurons in males have been less desensitized, which does not explain the decreased CO2 response. However, a reduction in postsynaptic 5-HT receptor function has been described in the 5-HTT knockout (Qu et al. 2005). Were this greater in males, it could account for the reduced CO2 response, e.g. chemoreceptor neurons in the retrotrapezoid nucleus that express 5-HT2A receptors could be less responsive to 5-HT release (Li et al. 2006; Mulkey et al. 2007).
Another unexpected observation is the greater 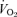 present at rest in 5-HTT knockout mice of both sexes. For this we have no clear explanation. The experiments were conducted at 24°C, which is below the thermoneutral zone of the mouse and should increase
present at rest in 5-HTT knockout mice of both sexes. For this we have no clear explanation. The experiments were conducted at 24°C, which is below the thermoneutral zone of the mouse and should increase 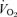 but in WT as well as knockout. Noradrenaline (NA) levels are unchanged in 5-HTT knockout mice under resting conditions but can show an enhanced response to stress (Tjurmina et al. 2002; Kim et al. 2005). Our mice are studied unrestrained in a chamber in which they can freely move about, certainly a stress much less than that reported to affect NA levels (Tjurmina et al. 2002) although predator odours alone can produce stress in 5-HTT knockouts (Adamec et al. 2006). The 5-HTT knockout mice could also have a thermoregulatory defect as shown, for example, in the Lmx1b conditional knockout mice (Hodges et al. 2008). Whatever the cause of the increased
but in WT as well as knockout. Noradrenaline (NA) levels are unchanged in 5-HTT knockout mice under resting conditions but can show an enhanced response to stress (Tjurmina et al. 2002; Kim et al. 2005). Our mice are studied unrestrained in a chamber in which they can freely move about, certainly a stress much less than that reported to affect NA levels (Tjurmina et al. 2002) although predator odours alone can produce stress in 5-HTT knockouts (Adamec et al. 2006). The 5-HTT knockout mice could also have a thermoregulatory defect as shown, for example, in the Lmx1b conditional knockout mice (Hodges et al. 2008). Whatever the cause of the increased 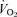 , the 5-HTT knockout mice respond with an appropriate increase in
, the 5-HTT knockout mice respond with an appropriate increase in 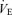 such that the ventilatory equivalent, the
such that the ventilatory equivalent, the 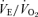 ratio, is unchanged from control. In a similar fashion, in response to the stress of hypoxia, the 5-HTT knockout mice responded with a normal
ratio, is unchanged from control. In a similar fashion, in response to the stress of hypoxia, the 5-HTT knockout mice responded with a normal 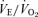 even though they had greater
even though they had greater 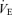 and
and 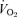 than did the WT controls. The 5-HTT knockout mice have a normal overall response to hypoxia.
than did the WT controls. The 5-HTT knockout mice have a normal overall response to hypoxia.
Conclusions
The 5-HTT knockout mice develop in the presence of excess ECF 5-HT. Initially this causes an excess of 5-HT ‘tone’; with time, there are adaptations that include down-regulation of 5-HT1A autoreceptors and variable adaptations in postsynaptic receptors. The time course of this switch from excess 5-HT function to reduced 5-HT function is unclear and may vary by sex, age and brain location. In adult male rats, daily microdialysis of the 5-HTT inhibitor fluoxetine into the medullary raphe for 3 weeks enhanced the CO2 response (Taylor et al. 2004) suggesting that with this dose and duration of treatment, the excess 5-HT was excitatory. With absence of 5-HTT function since early development, by adulthood there is most clearly a dramatic reduction of the CO2 response that is more prominent in males indicating that over this time period the net effect was a down-regulation of 5-HT function in chemoreception. This has relevance for SIDS in which (1) there are abnormalities in brainstem 5-HT neurons, (2) there is a male predominance, and (3) an important hypothesis for cause of death involves inadequate responses to asphyxia, which involve chemoreception (Thach, 2008). Our findings may also have relevance to the behavioural phenotype of the 5-HTT knockout mice which includes increased anxiety, lowered aggression and enhanced stress responses (Tjurmina et al. 2002; Holmes et al. 2003). One cannot study 5-HT function in physiology without reference to sex.
Acknowledgments
This work was supported by NHLBI grant R37 HL 28066.
References
- Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav Brain Res. 2006;170:126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Mitchell GS. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol. 2002;131:65–77. doi: 10.1016/s1569-9048(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Bouali S, Evrard A, Chastanet M, Lesch KP, Hamon M, Adrien J. Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur J Neurosci. 2003;18:2203–2212. doi: 10.1046/j.1460-9568.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Center for Health Statistics. [March 17, 2008];2008 CDC WONDER On-line Database, compiled from Compressed Mortality File 1999–2005 Series 20. No. 2K. Accessed at http://wonder.cdc.gov/cmf-icd10.html.
- Feldman JR, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Murphey DL, Lesch KP, Blier P. Modifications of the serotonergic system in mice lacking serotonin transporters: An in vivo electrophysiological study. J Pharm Exper Ther. 2001;296:987–995. [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol. 2004;97:2303–2309. doi: 10.1152/japplphysiol.00645.2004. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GT, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen Z-F, Richerson GB. Defects in breathing and thermoregulation in mice with near complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Jacono FJ, Peng YJ, Kumar GK, Prabhakar NR. Modulation of the hypoxic sensory response of the carotid body by 5-hydroxytryptamine: role of the 5-HT2 receptor. Respir Physiol Neurobiol. 2005;145:135–142. doi: 10.1016/j.resp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol. 2006;577:307–318. doi: 10.1113/jphysiol.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci. 2000;20:7868–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception 2005: a brief review. Auton Neurosci. 2006;126–127:332–338. doi: 10.1016/j.autneu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurons and adjacent neurons that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Activation of 5-HT1A receptors in rostral medullary raphe inhibits cutaneous vasoconstriction elicited by cold exposure in rabbits. Brain Res. 2006;1073–1074:252–261. doi: 10.1016/j.brainres.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J Appl Physiol. 2006;101:1177–1188. doi: 10.1152/japplphysiol.00376.2006. [DOI] [PubMed] [Google Scholar]
- Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 2005;180:12–20. doi: 10.1007/s00213-005-2231-5. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Samprado A. Homing in on the specific phenotype (s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Green A, Kinney HC, Nattie EE. Chronic fluoxetine microdialysis into the medullary raphe nuclei of the rat, but not systemic administration increases the ventilatory response to CO2. J Appl Physiol. 2004;97:1763–1773. doi: 10.1152/japplphysiol.00496.2004. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach B. Tragic and sudden death. Potential and proven mechanisms causing sudden infant death syndrome. EMBO Rep. 2008;9:114–118. doi: 10.1038/sj.embor.7401163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphey DL. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology. 2002;143:4520–4526. doi: 10.1210/en.2002-220416. [DOI] [PubMed] [Google Scholar]


