Abstract
Despite the enormous diversity of glutamate (Glu) receptors and advances in understanding recombinant receptors, native Glu receptors underlying functionally identified inputs in active systems are poorly defined in comparison. In the present study we use UBP-302, which antagonizes GluR5 subunit-containing kainate (KA) receptors at ≤ 10 μm, but other KA and AMPA receptors at ≥ 100 μm, and rhythmically active in vitro preparations of neonatal rat to explore the contribution of non-NMDA receptor signalling in rhythm-generating and motor output compartments of the inspiratory network. At 10 μm, UBP-302 had no effect on inspiratory burst frequency or amplitude. At 100 μm, burst amplitude recorded from XII, C1 and C4 nerve roots was significantly reduced, but frequency was unaffected. The lack of a frequency effect was confirmed when local application of UBP-302 (100 μm) into the pre-Bötzinger complex (preBötC) did not affect frequency but substance P evoked a 2-fold increase. A UBP-302-sensitive (10 μm), ATPA-evoked frequency increase, however, established that preBötC networks are sensitive to GluR5 activation. Whole-cell recordings demonstrated that XII motoneurons also express functional GluR5-containing KA receptors that do not contribute to inspiratory drive, and confirmed the dose dependence of UBP-302 actions on KA and AMPA receptors. Our data provide the first evidence that the non-NMDA (most probably AMPA) receptors mediating glutamatergic transmission within preBötC inspiratory rhythm-generating networks are pharmacologically distinct from those transmitting drive to inspiratory motoneurons. This differential expression may ultimately be exploited pharmacologically to separately counteract depression of central respiratory rhythmogenesis or manipulate the drive to motoneurons controlling airway and pump musculature.
Fast excitatory synaptic transmission in the mammalian CNS is mediated predominately by glutamate (Glu) acting at AMPA, kainate (KA) and NMDA subtypes of ionotropic Glu receptors (Kullmann, 2001). Molecular analyses have revealed that Glu receptor diversity greatly exceeds that suggested by this early pharmacological classification. Understanding the significance of Glu receptor diversity for brain function is an important objective of modern neuroscience from both basic and clinical perspectives. Indeed, there is a long-standing, but largely unrealized hope that understanding the diversity of Glu receptors and their spatiotemporal patterns of expression in the brain will provide the foundation for development of specific pharmacotherapies. Understanding of recombinant Glu receptors far exceeds that of native receptors in identified, active pathways, reflecting in part a dearth of subunit-selective antagonists. Even when the relative abundance of subunits within a cell type is established, this does not define which subunits combine to form native receptors, nor how the different subtypes are distributed to functionally distinct synapses. Thus, the majority of work investigating the physiological role of ionotropic Glu receptor subtypes only distinguishes between the relative contributions of AMPA and NMDA receptors. The functional significance of KA receptors is most poorly understood, but a diversity of roles is emerging (Wisden & Seeburg, 1993; Cossart et al. 1998; Li et al. 1999; Kullmann, 2001; Liu et al. 2004), including modulation of synaptic transmission and plasticity (Bortolotto et al. 1999; Sallert et al. 2007) and regulation of synaptic inhibition (Clarke et al. 1997).
Progress has been facilitated by the development of willardine derivatives (Patneau et al. 1992). One such compound, UBP-302, antagonizes GluR5 subunit-containing KA receptors at low concentrations, but other KA and AMPA receptors at higher concentrations (More et al. 2004; Mayer et al. 2006). Here we use this compound to explore glutamatergic signalling in central respiratory networks of the neonatal rat, where AMPA receptors play a prominent role in inspiratory rhythm generation, and the transmission of inspiratory drive to motoneurons (McCrimmon et al. 1989; Greer et al. 1991; Funk et al. 1993, 1997; Ge & Feldman, 1998). Neither the subunit composition of these AMPA receptors, nor whether rhythm-generating and motor output components of this network use identical AMPA receptors is known. Moreover, although inspiratory motoneurons and neurons within the ventrolateral medulla strongly express GluR5–7 and KA1–2 subunits (Robinson & Ellenberger, 1997; Garcia Del Cano et al. 1999; Paarmann et al. 2000), the involvement of KA receptors in respiratory control is unknown. We therefore used in vitro preparations in which endogenously released Glu rhythmically activates synaptic receptors that mediate inspiratory activity, and exploited the dose-dependent actions of UBP-302 to test the differential contribution of GluR5 receptors to glutamatergic signalling in rhythm-generating and motor output compartments of the inspiratory network. We also tested whether the AMPA receptors in the rhythmogenic preBötC and inspiratory motoneuron pools are differentially sensitive to UBP-302. Our data in the neonatal rat in vitro indicate that while preBötC rhythm is sensitive to GluR5 activation and XII motoneurons express functional GluR5 subunit-containing KA receptors, these receptors do not contribute to endogenous rhythm nor motor output in vitro. Data also indicate that the non-NMDA (most probably AMPA) receptors mediating drive to motoneurons are distinct from those in preBötC rhythm-generating networks. These basic data have implications for the development of drug therapies to separately promote inspiratory rhythmogenesis or activate inspiratory musculature in prematurity, disease and drug-induced respiratory depression.
Methods
Ethical approval
All experiments and procedures were approved by the University of Alberta Animal Ethics Committee and performed in accordance with their guidelines for the care, handling and treatment of experimental animals.
Brainstem–spinal cord and medullary slice preparations
Preparations were obtained from postnatal day (P) 0–3 (brainstem–spinal cord) or P0–4 (medullary slice) Wistar rats using methods previously described (Miles et al. 2002; Lorier et al. 2007). Briefly, animals were anaesthetized with isoflurane and decerebrated. The brainstem–spinal cord was isolated in artificial cerebrospinal fluid (aCSF) containing (mm): 120 NaCl, 3 KCl, 1.0 CaCl2, 2.0 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, and 20 d-glucose, pH 7.4, equilibrated with 95% O2–5% CO2, at 20–22°C. For the brainstem–spinal cord, preparations extended from the caudal-pontine level rostrally to approximately the 7th cervical segment caudally. Once dissected free, the brainstem–spinal cord was pinned down with the ventral surface up on Sylgard resin in a recording chamber (10 ml) perfused with aCSF (10 ml min−1) and aerated with 95% O2–5% CO2.
To obtain rhythmic medullary slice preparations the brainstem–spinal cord was isolated as described above and then pinned to a wax chuck and sectioned using a vibratome (Leica VT1000S). A series of 100–200 μm sections was cut until the compact division of nucleus ambiguus (cNA) was no longer visible in these thin sections when trans-illuminated, and the rostral margin of the inferior olive first appeared. A 700 μm transverse slice was then cut, which extended from −0.3 to −1.0 mm caudal to the facial nucleus as described in the online atlas (Ruangkittisakul et al. 2006) perfused with aCSF bubbled with 95% O2–5% CO2 at a flow rate of 10–12 ml min−1. Thirty minutes prior to the start of data collection the extracellular K+ in the circulating aCSF was increased to 9 mm as this provided > 7 h of stable inspiratory network activity (Smith et al. 1991; Funk et al. 1993). Note that while the increase in K+ will shift its reversal potential, the shift will have a similar effect on currents through all Glu receptor subtypes.
The bath temperature was gradually increased for both preparations over 30 min from room temperature to 27–28°C before recording. C4/C4 or C4/C1 (brainstem–spinal cord) or XII (medullary slice) nerve activities were recorded bilaterally using suction electrodes with internal tip diameters ranging from 80 to 100 μm. C4 motoneurons innervate the diaphragm, C1 motoneurons innervate accessory respiratory muscles (Kitamura & Sakai, 1982; Ullah et al. 2007), and XII motoneurons innervate tongue musculature. Thus, we examined glutamatergic drive to three sets of inspiratory motoneurons. Recordings were amplified (50 000 times), band-pass filtered (0.1–3 kHz), full wave rectified and integrated (τ= 50 ms) (Model 1700 Differential AC Amplifier, A-M Systems, Everett, WA, USA; MA 821/RSP; CWE, Ardmore, PA, USA). Raw and integrated nerve activities were recorded using Axon Instruments Digidata 1322 A/D board and Axoscope (v. 9.2), and stored on computer for off-line analysis.
Whole-cell recordings
Whole-cell recordings of XII motoneurons were obtained from 300 μm, non-rhythmic medullary slices. The brainstem–spinal cord was dissected as described above, glued rostral surface down to a metal plate and an agar block situated behind the tissue to provide support. Tissue was then serially sectioned in the caudal-to-rostral direction using a vibratome (Leica, VT1000S). Three to four, 300 μm slices containing the XII nucleus were obtained per neonate and incubated at 35°C in aCSF for 40 min, followed by 1 h at room temperature in fresh aCSF (Aghajanian & Rasmussen, 1989; Ireland et al. 2004).
Slices were then placed in the bath of a temperature-controlled (TC-1 temperature controller, Bioscience Tools, San Diego, CA, USA) recording chamber (2 ml, 27°C) and perfused with oxygenated aCSF at a rate 2 ml min−1. The chamber (Bioscience Tools) was mounted on the fixed stage of an upright microscope equipped with IR-DIC optics (Zeiss Axioskop 2 FS Plus). Recordings from XII motoneurons were established under direct visualization using IR-DIC optics, a CCD camera (IR-1000, Dage-MTI, Michigan City, IN, USA) and monitor (M910; National Electronics) attached to the microscope. The XII nucleus is a relatively homogeneous nucleus, in which XII motoneurons are easily identified as < 10% of neurons are interneurons (Viana et al. 1993). Glass micropipettes (3–4.5 MΩ) were pulled on a horizontal puller (model P-97; Sutter Instruments, Novato, CA, USA) from 1.2 mm OD borosilicate glass (World Precision Instruments, Sarasota, FL, USA) and filled with a potassium gluconate-based solution containing (mm): 122.5 potassium gluconate, 17.5 KCl, 9 NaCl, 1 MgCl2, 10 Hepes and 0.2 EGTA, pH adjusted to 7.3 (with 5 m KOH). Patch pipettes were lowered under positive pressure along the z-axis onto a previously selected XII motoneuron until a ‘dimple’ was observed in the neuron membrane. Pressure was then switched rapidly from positive to negative and a gigaohm seal formed almost immediately (typically 3–5 GΩ). The whole-cell configuration was then obtained by gently applying negative pressure pulses.
Intracellular signals were amplified and filtered (2 kHz low-pass Bessel filter) with a Multiclamp 700B amplifier (Axon Instruments, Union City, CA, USA) and acquired via a Digidata 1322A analog-to-digital board and pCLAMP 9.2 software. Series resistance and whole-cell capacitance were estimated under voltage-clamp conditions using short voltage pulses (100 Hz, 10 mV, 3 ms). Series resistance was monitored throughout the experiments and results were discarded if it changed by more than 10% between control, test and recovery trials. All whole-cell recordings were performed in the presence of tetrodotoxin (TTX, 0.5 μm). Neuronal input resistance (RN) was determined in a subset of motoneurons by measuring the current response to square-wave, −10 mV hyperpolarizing voltage pulses (400 ms) delivered before, during and after drug application.
Drugs and their application
Drugs used included: (+/–)-α-amino-3-hydroxy-5-methy-lisoxazole-4-propionic acid hydrate (AMPA, 100 μm, Sigma, St Louis, MO, USA); (RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA, 100 μm, Tocris, Ellsville, MO, USA); (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxybenzyl) pyrimidine-2,4-dione (UBP-302, 10–100 μm, Tocris); (+/–)-4-(4-aminophenyl)-1,2-dihydro-1-methyl-2-propylcarbamoyl-6,7-methylenedioxyphthalazine (SYM 2206, 10–100 μm, Tocris); (TTX, 0.5 μm, Alomone Laboratories, Israel); [sar9-met(O2)11]-Substance P (SP, 1 μm, Tocris). All drugs were dissolved in standard aCSF, except SYM 2206 which was dissolved in DMSO. The final concentration of DMSO never exceeded 0.1%. We have established previously that DMSO is without non-specific actions on membrane or channel properties at concentrations up to 0.5% (Funk et al. 1995). SYM 2206 and TTX were added to the perfusate, UBP-302 was added to the perfusate or applied locally, while AMPA, ATPA and SP were delivered by local application.
Local application of drugs was performed via pressure injection using triple-barrelled drug ejection pipettes constructed so that each barrel was between 6 and 7 μm (outside tip diameter). Larger pipettes were discarded. Injection protocols were controlled via a programmable stimulator (Master-8, AMPI, Israel or Digitimer, type 3290). For whole-cell recording, drug pipettes were positioned above the tissue within 20 μm (in the x–y plane) of the XII motoneuron under investigation. Local injection within the preBötC was established by first mapping the ventrolateral medulla for the site that gave the strongest response to local application of SP (Gray et al. 1999; Lorier et al. 2007). Drugs were delivered into the tissue at 34 kPa(5 p.s.i.) or above the tissue at 69 kPa(10 p.s.i.) (whole-cell recording). Please note that the concentrations of drugs used in the present study should not be directly compared with those in experiments where similar agents are applied in the bath or directly to isolated cells. Firstly, the concentration of drug decreases exponentially with distance from the pipette tip (Nicholson, 1985) and previous experiments with this preparation have established that drug concentration in the pipette must be approximately 10-fold greater than the bath-applied concentration to produce similar effects (Liu et al. 1990). Secondly, diffusion barriers present in thick slices slow response kinetics relative to isolated cells. Thus, for local application protocols as used here, the concentration of drug at the site, or neuron, of interest will gradually increase over time to a steady-state concentration less than that in the pipette. In whole-cell experiments, when the pipette is above the tissue and agonist (AMPA and ATPA) applications range between 0.1 and 2 s, this steady state is not reached, as shown by the observation that current amplitude increases with the duration of drug application. Some variation in the drug concentration achieved is inevitable between experiments for several reasons including minor variations in barrel diameter, as well as differences in the spatial relationship between pipette tip and the neuron (including the cell depth) and flow dynamics in the chamber. Such variability was minimized by rigorously standardizing application parameters (consistent aCSF flow rates and direction, pipette position, constant pressure and duration, pipettes selected for consistent geometry).
There can also be variation between consecutive applications within an experiment, primarily reflecting pipette blockage. This is minimal when injecting above tissue as done here for whole-cell recording. Blockage can be more problematic when injecting into tissue. However, our experience with injection into medullary slices and brainstem–spinal cords of neonatal rodents in vitro is that if pia mater is removed, the injection volume typically varies < 10%. We did not measure the volume of every injection, but pipette patency was checked before and after every experiment. Data were discarded if blockage was apparent. Volume ejected into the tissue was measured in a separate calibration procedure by measuring the movement of meniscus in response to pressure pulses of different durations. Volumes injected into tissue during injections of 10, 15 or 90 s duration were approximately 8, 12 and 75 nl, respectively. Volumes ejected above the tissue were not measured but are likely to be similar. Volumes associated with 0.1–2 s ejections were not measurable.
Data analysis
The amplitude of agonist-evoked currents was determined by subtracting the baseline current averaged over a 5–10 s period immediately preceding agonist application from the peak of the agonist-induced current. For bath application of drugs to rhythmic brainstem–spinal cord and medullary slice preparations, responses were calculated by averaging inspiratory burst frequency and amplitude in 2 min bins for the 10 min prior to drug application (control period), throughout drug application and for 30 min during drug washout. Data are reported relative to the average value during the control period that immediately preceded drug application. To analyse the effects on frequency and amplitude of locally applying drugs to the preBötC, parameters were compared to the average value during the 90 s control period that immediately preceded drug application. The maximum effect of a drug on frequency or amplitude was determined as the maximum (or minimum) value in the moving average calculated from four consecutive cycles during the first minute after the start of drug injection. Data are reported as means ±s.e.m. Differences between means were assessed on raw data using ANOVA with Newman–Keuls multiple comparison test. With the exception of datasets with n = 4 (which could not be tested), data satisfied the Kolmogorov–Smirnov test for normality (Graphpad Prism 4.2). Differences between means were considered significant when P < 0.05.
Results
Effects of UBP-302 on network activity
The first objective was to assess the role of GluR5 subunit-containing KA receptors in inspiratory rhythm generation and transmission of this drive to cranial (XII) and spinal (C1 and C4) inspiratory motoneurons. We therefore tested the effects of bath applying 10 μm UBP-302 on inspiratory frequency and burst amplitude generated by brainstem–spinal cord and medullary slice preparations. UBP-302 selectively antagonizes GluR5 subunit-containing KA receptors at concentrations ≤ 10 μm, but also antagonizes AMPA receptors at concentrations ≥ 100 μm (More et al. 2004; Mayer et al. 2006). Inspiratory frequency recorded from C1 and C4 nerve roots in brainstem–spinal cord preparations (Fig. 1) or XII nerves in medullary slice preparations (Fig. 2) was unaffected by the lower, GluR5-selective concentration of UBP-302. Inspiratory burst amplitude recorded from C1, C4 (Fig. 1) and XII nerves (Fig. 2) measured 0.76 ± 0.08 (n = 4), 0.97 ± 0.04 (n = 5) and 0.82 ± 0.07 (n = 5) of control, respectively. None of these changes reached significance.
Figure 1. UBP-302 (100 μM) inhibits spinal inspiratory burst amplitude but not frequency.
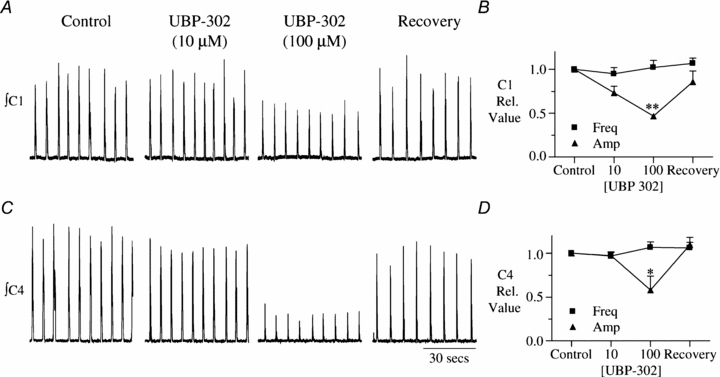
UBP-302 effects on integrated C1 (A) and C4 (C) nerve bursts in the presence of low (10 μm), high (100 μm) UBP-302 and following washout. B and D, group summary data (n = 5). Asterisk indicates significance: *P < 0.05; **P < 0.001.
Figure 2. UBP-302 (100 μM) inhibits XII inspiratory burst amplitude but not frequency.
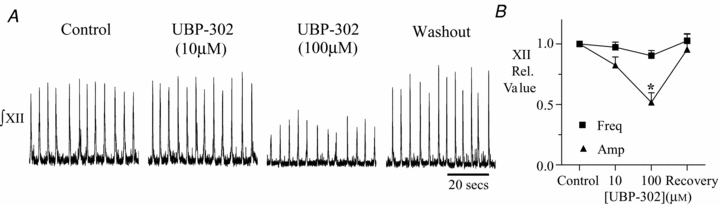
A, XII nerve recordings from a medullary slice preparation showing the response to bath application of 10 and 100 μm UBP-302. B, group data showing the reversible attenuation of XII burst amplitude in 100 μm UBP-302. XII burst frequency remains unaffected. Asterisk indicates significance, *P < 0.05.
Increasing the concentration of UBP-302 in the bath to 100 μm was also without effect on frequency in both the brainstem–spinal cord (n = 5) and medullary slice (n = 5) preparations (Figs 1 and 2). In the brainstem–spinal cord preparation, frequency was 9.8 ± 0.8 bursts min−1 in control and 9.5 ± 1.1 bursts min−1 in 100 μm (n = 5; Fig. 1A). In the slice preparation, frequency was 14 ± 1.3 and 13 ± 1.4 bursts min−1 in control and 100 μm UBP-302, respectively, i.e. 0.90 ± 0.04 of control (n = 5; Fig. 2). In contrast, 100 μm UBP-302 significantly reduced inspiratory burst amplitude in all nerves. C1, C4 and XII burst amplitudes decreased to 0.47 ± 0.02 (n = 4; P < 0.001; Fig. 1A and B), 0.58 ± 0.16 (n = 5; P < 0.05; Fig. 1C and D), and 0.51 ± 0.07 of control (n = 5; P < 0.05; Fig. 2A and B), respectively.
We next explored the possibility that the apparent differential sensitivity of burst amplitude and frequency to UBP-302 might reflect greater access of the drug to superficially located motoneuron pools compared to rhythm-generating networks located deeper within the tissue. In the brainstem–spinal cord preparation for example, phrenic motoneurons that produce the inspiratory activity on the C4 nerve are within 200 μm of the surface (Lindsay et al. 1991), while rhythm-generating networks are between 300 and 500 μm from the surface (Smith et al. 1991; Funk et al. 1993; Ruangkittisakul et al. 2006). In the slice, preBötC rhythm-generating networks and XII motoneuron pools are both located superficially. Thus, differential diffusion of UBP-302 is less likely than in the brainstem–spinal cord preparation. Nevertheless, to confirm that UBP-302 has no effect on inspiratory rhythm-generating networks, we locally injected UBP-302 (100 μm) directly into the preBötC.
The preBötC was located by its sensitivity to local application of the NK1 receptor agonist [sar9-met(O2)11]-Substance P (SP; 1 μm, 10 s). A high proportion of preBötC neurons express NK1 receptors (Gray et al. 1999, 2001). Indeed, high levels of NK1 receptor immunolabelling is the most widely used anatomical criterion to identify the location of the preBötC (Pagliardini et al. 2005). Functionally, a consequence of the high NK1 receptor expression in the preBötC is that activation of NK1 receptors in this region evokes a significant increase in inspiratory frequency. In our experiments, local application of SP (1 μm, 10 s) into the SP-sensitive region evoked an increase in inspiratory frequency that peaked on average at 2.04 ± 0.3-fold greater than baseline (n = 4; P < 0.05; Fig. 3A and B). Local application of UBP-302 (100 μm, 10 s) into the preBötC site where SP evoked this frequency increase had no significant effect on XII nerve burst frequency or amplitude, which averaged 1.06 ± 0.04 and 0.95 ± 0.04 of control (n = 4; Fig. 3C and D) during the 1 min period following the onset of UBP-302 application.
Figure 3. GluR5 subunit-containing KA receptors do not contribute to endogenous inspiratory-related rhythm.
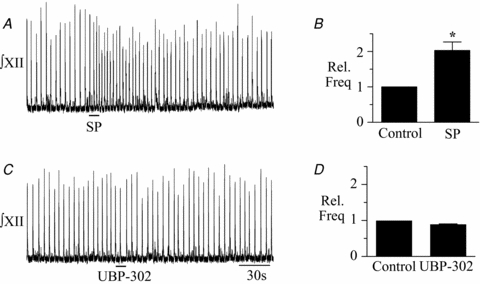
A, SP (1 μm) application elicits an increase in frequency verifying correct placement of drug pipette within the rhythmogenic preBötC. B, group data showing the peak increase in XII nerve burst frequency evoked by local application of SP into the preBötC (n = 4). C, local application of UBP-302 (100 μm) from the same drug pipette into the same site that SP was injected in A had no effect on frequency. D, group data showing the average burst frequency during control and the minimum frequency observed in the 60 s following local injection of UBP-302. Asterisk indicates significance, *P < 0.05.
Finally, we tested whether preBötC networks are sensitive to GluR5 receptor activation. We locally applied ATPA (10 and 100 μm) into the preBötC and evoked a dose-dependent increase in frequency from 13.6 ± 1.1 bursts min−1 in control to 20.9 ± 2.1 bursts min−1 (1.56 ± 0.13-fold) and 44.9 ± 4.8 bursts min−1 (3.42 ± 0.54-fold), respectively (Fig. 4, n = 5). Most relevant in terms of establishing GluR5 sensitivity, however, is that the significant 1.56 ± 0.13-fold increase in frequency evoked by 10 μm ATPA in control was significantly reduced to 1.12 ± 0.09 by 10 μm UBP-302.
Figure 4. PreBötC rhythm-generating circuits are sensitive to activation of GluR5 subunit-containing KA receptors.
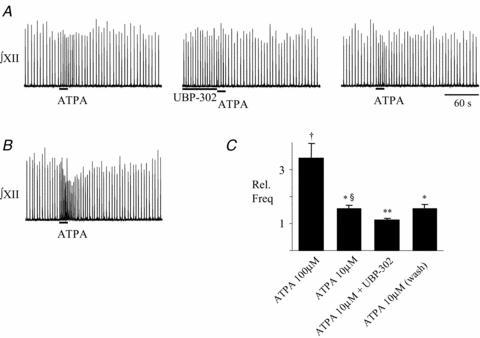
A, local unilateral application of ATPA (10 μm, 15 s) into the preBötC in control, immediately after 90 s pre-application of UBP-302 (10 μm) 15 min later, and following 15 min of washout. B, application of 100 μm ATPA into the same site as in A, showing dose dependence of the ATPA-evoked frequency response. C, group data (n = 5) showing relative effects on frequency of applying into the preBötC 100 μm ATPA (15 s) alone, and 10 μm ATPA in control, after 90 s pre-application of 10 μm UBP-302 and after washout of UBP-302 for 15 min † indicates Significant difference from control, P < 0.001; * indicates significant difference from control and 100 μm ATPA, P < 0.001; ** indicates significant difference from control, P < 0.05; § indicates significant difference from ATPA + UBP-302, P < 0.05.
Effects of UBP-302 on KA and AMPA receptor-mediated currents
Our objectives with this series of experiments were to use whole-cell recording to test for the presence of functional GluR5 subunit-containing KA receptors on XII motoneurons, but more importantly to assess whether the relative selectivity of UBP-302 for GluR5-containing KA receptors and AMPA receptors defined previously also applied in the rhythmic slices. An important technical note is that these experiments do not define the absolute magnitude of AMPA or ATPA currents. Nor do they allow direct comparison of AMPA versus ATPA current amplitudes. The rate of drug delivered from the different pipette barrels was the same but the duration, and therefore the concentration reached at the motoneuron, was agonist dependent (longer duration applications were required to evoke measurable currents with ATPA).
We assessed in TTX the magnitude of KA receptor-mediated currents and their sensitivity to KA receptor antagonists. Local application of the KA receptor agonist, ATPA (100 μm; 0.5–1 s), elicited small inward currents that averaged −23 ± 4.5 pA (n = 4, Fig. 5A) in motoneurons held at −60 mV. ATPA-evoked currents were significantly reduced to −13.5 ± 1.4 pA (P < 0.05) and −6.4 ± 1.6 pA (P < 0.01) by the bath application of 10 and 100 μm UBP-302, respectively (Fig. 5A and B).
Figure 5. ATPA-mediated currents are antagonized by the GluR5 receptor antagonist UBP-302 in XII motoneurons.
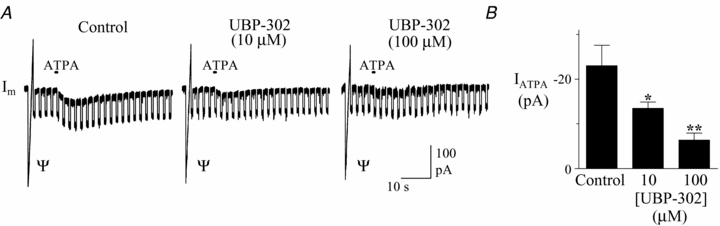
A, membrane current responses to local application of ATPA (500 ms) in the presence of control, 10 and 100 μm UBP-302. B, pooled data of responses showing the inhibition of ATPA-mediated currents by UBP-302 at 10 and 100 μm (n = 4). Square-wave steps are voltage pulses to test membrane resistance, which did not change during these small currents. Ψ indicates current response to voltage ramp. Asterisks indicate significant difference from control currents, *P < 0.05, **P < 0.01.
ATPA is more than 100-fold more selective for GluR5-containing KA receptors than other KA and AMPA receptors (Clarke et al. 1997), but the EC50 for ATPA at these other receptors is only 3- to 4-fold higher than used here. Thus, ATPA currents may include a small non-GluR5 receptor component. Similarly, although affinity of UBP-302 for GluR5 receptors is 100- to 1000-fold higher than for other non-NMDA receptors, analysis of recombinant Glu receptors indicates that 100 μm UBP-302 can also antagonize AMPA receptors (More et al. 2004; Mayer et al. 2006). Indeed, in XII motoneurons, −197 ± 32 pA currents evoked by local application of AMPA (100 μm, 0.1 s) were significantly reduced by 100 μm (−110 ± 28 pA, P < 0.05; n = 6, data not shown) but not 10 μm UBP-302 (−178 ± 39 pA). As AMPA has an EC50 for GluR5-containing KA receptors in the 100 μm range (Alt et al. 2004), these actions of UBP-302 could reflect antagonism of AMPA-evoked, GluR5 KA, or AMPA receptor currents. The next objective was therefore to test the effects of UBP-302 on the ATPA-evoked current after any potential AMPA receptor-mediated component was blocked. First, we established in TTX the concentration of the non-competitive AMPA receptor antagonist, SYM 2206, required to block AMPA currents. SYM 2206 was used because it is more selective for AMPA over KA receptors compared to other AMPA antagonists. SYM 2206 for example, has an IC50 of 2.8 μm at AMPA receptors while at 100 μm it blocks only 10% of KA receptor responses (Pelletier et al. 1996). In neonatal rat spinal cord, the IC50 values for NBQX at AMPA and KA are receptors are 0.2 and 3.16 μm, respectively (Zeman & Lodge, 1992). In the first series, 10 μm SYM 2206 caused a significant, but incomplete, 46% reduction of AMPA currents (100 μm; 0.1–0.4 s) from −216 ± 52 pA to −116 ± 28 pA (n = 3; Fig. 6A). In another sample of neurons we therefore increased the concentration of SYM 2206 and established that at 100 μm, SYM 2206 reduced control AMPA currents from −261 ± 21 pA to −16.3 ± 6.5 pA (P < 0.001; n = 8; Fig. 6B), i.e. to 6% of the control current.
Figure 6. Attenuation of AMPA-mediated currents by SYM 2206 (10 or 100 μM) in XII motoneurons.
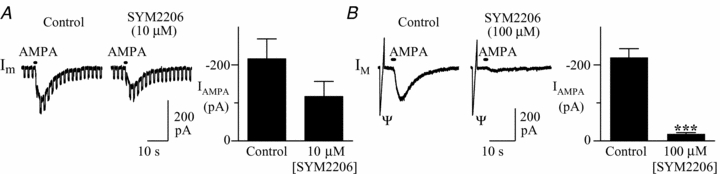
A, membrane current responses to local application of AMPA in control and SYM 2206 (10 μm). Pooled data showing a partial, but insignificant, reduction of AMPA-mediated currents in the presence of SYM 2206 (10 μm) (n = 3). Square-wave steps are voltage pulses delivered to test membrane resistance. B, membrane current response from a XII motoneuron showing a reduction in the AMPA-mediated current by SYM 2206 (100 μm). Pooled data showing a significant reduction of AMPA currents in SYM 2206 (Student's paired t test; n = 8). Ψ indicates current response to voltage ramp. Asterisks indicate significance, ***P < 0.001.
Having established that 100 μm SYM 2206 virtually blocks AMPA currents, we performed a more definitive test for the presence of functional GluR5 subunit-containing KA receptors on XII motoneurons by examining, in the presence of 100 μm SYM 2206, the effects of UBP-302 on ATPA currents. In this series, ATPA was applied locally for 2 s to evoke larger currents than in the previous experiments. ATPA currents averaged −51.1 ± 6.2 pA (n = 6; Fig. 7A and B). Bath application of SYM 2206 at 100 μm on average reduced these currents to −32.4 ± 10.3 pA (P < 0.05); however, in some cells like that shown in Fig. 7A, SYM 2206 had minimal effect on the ATPA current. On average, the SYM 2206-insensitive ATPA currents were reduced from −32 ± 10 pA to −8.5 ± 2.7 pA by 10 μm UBP-302 (n = 6; Fig. 7A and B). UBP-302 at 100 μm caused a further numerical reduction in ATPA current amplitude to −1.7 ± 1.5 pA, but this change was not statistically significant. Note also in Fig. 7A, that the SYM 2206-insensitive ATPA current is completely blocked by 10 μm UBP-302.
Figure 7. Attenuation of ATPA-mediated currents by SYM 2206 (100 μM) and UBP302 (10–100 μM) in XII motoneurons.
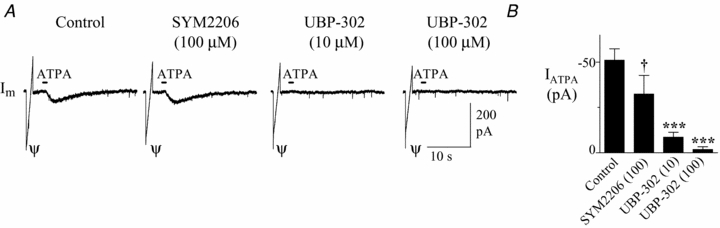
A, membrane current responses to ATPA application in the presence of control, SYM 2206 (100 μm), UBP 302 (10 μm) and UBP 302 (10 μm). B, pooled data showing a significant reduction of ATPA currents by SYM 2206 at 100 μm, and a further reduction that was similar for 10 and 100 mm UBP 302 (n = 6). † indicates significant difference from the control ATPA current (P < 0.05). Asterisks indicate significant difference from the ATPA current evoked in 100 μm SYM 2206, ***P < 0.001.
Discussion
In this study we have used a pharmacological approach to demonstrate in neonatal rat that there are distinct Glu, most probably AMPA, receptor subtypes involved in inspiratory rhythm generation and the transmission of inspiratory drive to motoneurons. We also demonstrate that while functional GluR5 subunit-containing KA receptors can modulate rhythmogenic preBötC networks and are present on XII motoneurons, they do not contribute to the generation of inspiratory rhythm nor the transmission of inspiratory drive to XII and phrenic motoneurons in vitro.
The complement of Glu receptors used by inspiratory rhythm-generating networks and drive transmission pathways are not identical
Glutamatergic transmission is fundamental to both the generation of inspiratory rhythm within the preBötC and the transmission of this rhythmic drive to inspiratory motoneurons within the brainstem and spinal cord (McCrimmon et al. 1989; Greer et al. 1991; Smith et al. 1991; Funk et al. 1993; Feldman & Del Negro, 2006). Recent data have revealed significant roles for NMDA and group I metabotropic Glu receptors in mediating inspiratory drive potentials in preBötC neurons (Pace et al. 2007). In contrast, the importance of ionotropic non-NMDA receptors in rhythm generation and drive transmission in neonatal rodents has been established for almost 15 years (McCrimmon et al. 1989; Greer et al. 1991; Funk et al. 1993; Ge & Feldman, 1998). AMPA receptors are considered the main non-NMDA receptor contributor, but surprisingly this is based primarily on the greater sensitivity of respiratory neurons to AMPA versus KA agonists rather than on direct antagonist data. Similarly, the specific complement of AMPA or KA receptor subunits that mediate transmission in these two compartments of the central respiratory network is unknown.
We demonstrated that preBötC rhythm-generating networks are sensitive to GluR5 subunit-containing KA receptor activation. However, our observation that low concentrations of UBP-302 sufficient to antagonize ATPA-evoked KA currents had no effect on endogenous inspiratory activity suggests that GluR5 subunit-containing KA receptors do not contribute significantly to rhythmic inspiratory-related activity in neonatal rat in vitro. In addition, the observations that high concentrations of UBP-302 (100 μm): (1) reduced XII, C1 and C4 motor output; (2) attenuated AMPA- and ATPA-evoked currents in XII motoneurons; but (3) had no effect on inspiratory frequency in brainstem–spinal cord and medullary slice preparations, suggest differential expression of non-NMDA Glu receptor subtypes within inspiratory rhythm-generating networks and pathways transmitting this drive to inspiratory motoneurons. This does not mean that there is no overlap between the two populations, but data indicate that there is a UBP-302-sensitive pool of Glu receptors, most probably AMPA receptors, in motoneurons that is absent from rhythm-generating networks.
The strength of this conclusion, i.e. that there is differential expression of non-NMDA/AMPA receptors at inspiratory synapses in these two network compartments, depends on the pharmacological selectivity of the AMPA and KA receptor agonists and antagonists used. Most relevant is the dose-dependent selectivity of UBP-302 for GluR5 subunit-containing KA receptors at ≤ 10 μm and its additional antagonism of other KA and AMPA receptors at concentrations ≥ 100 μm, which has been demonstrated in both native receptors (More et al. 2004; Olsen et al. 2007) and recombinant receptors (Mayer et al. 2006).
Several observations suggest that UBP-302 acted with similar efficacy in our system. First, antagonizing GluR5 and AMPA receptors at ≤ 10 and ≥ 100 μm, respectively. First, selective inhibition of GluR5 currents by 10 μm UBP-302 is consistent with the sensitivity of a small portion of the AMPA (100 μm) current in XII motoneurons to 10 μm UBP-302 (∼19 pA; −197 ± 32 pA in control and −178 ± 39 pA in 10 μm UBP-302). The EC50 of AMPA for GluR5-containing KA receptors is in the 100 μm range (Alt et al. 2004). Thus, one would expect that currents evoked by 100 μm AMPA would include a small KA receptor component.
Second, when GluR5-mediated KA currents are isolated using ATPA in 100 μm SYM 2206 to remove the potential for ATPA-evoked AMPA currents (Clarke et al. 1997; Alt et al. 2004) (which is suggested by small SYM 2206-sensitive ATPA currents, Fig. 7), the antagonism by UBP-302 is not dose dependent; it is the same at 10 and 100 μm (Fig. 7). Similarly, the amplitude of the AMPA current that was blocked by 100 μm UBP-302 was ∼87 pA (−197 ± 32 pA in control compared to −110 ± 28 pA in UBP-302), double the ATPA current evoked in SYM 2206, suggesting that at 100 μm UBP-302 blocks AMPA currents as well. Taken together, these data, and the much greater affinity of ATPA for homo- or heteromeric GluR5 subunit-containing KA receptors over other KA subunits (GluR6, KA2) (Alt et al. 2004), are consistent with earlier data indicating that 10 μm UBP-302 will primarily affect GluR5 subunit-containing KA receptors, and that at ≥ 100 μm it will also affect AMPA receptors (More et al. 2004; Mayer et al. 2006; Olsen et al. 2007).
Differential modulation of rhythm and motor output by UBP-302: AMPA receptor subtypes
The molecular mechanisms underlying the differential sensitivity to UBP-302 of non-NMDA receptors involved in inspiratory rhythm generation and motor output are not known. It could reflect differences in subunit composition or post-transcriptional processing of gene transcripts (e.g. alternate splicing and RNA editing) in the preBötC and motor nuclei. Current understanding of the AMPA and KA receptors expressed in these two compartments of the inspiratory network, however, is insufficient to provide significant insight.
XII motoneurons and unidentified preBötC neurons express all AMPA and all KA receptor subunits with the sole exception of KA2. The splice variants flip and flop of the GluR1–4 subunits are also expressed in both cell groups (Petralia & Wenthold, 1992; Garcia Del Cano et al. 1999; Paarmann et al. 2000). Amongst the four types of RNA editing undergone by AMPA and KA receptor transcripts, only R/G editing of GluR2 has been examined via single-cell RT-PCR analysis, which revealed that XII motoneurons and preBötC interneurons express the R/G edited variant of the GluR2 subunit (Paarmann et al. 2000). The only notable difference documented thus far is that GluR7 subunit expression is higher in preBötC neurons compared to XII motoneurons (Paarmann et al. 2000), but the functional significance of this is unclear.
Given the difficulty of characterizing AMPA or KA receptor subtypes at inspiratory synapses in either compartment, an alternate approach to understand the differential actions of UBP-302 is to establish using expression systems the dose-dependent sensitivity of all AMPA and non-GluR5 KA receptors to UBP-302. This would also help establish the subunit composition of preBötC neurons and inspiratory motoneurons. However, data are incomplete. Affinity at GluR2, 5, 6 and KA2 subunits is established (see above), but the efficacy of UBP-302 at GluR1, GluR3, GluR4, GluR7 and KA1 subunits is not.
Contribution of GluR5 subunit-containing KA receptors to inspiratory network activity
KA receptor mRNA and protein have been localized both pre- and postsynaptically in numerous neuronal groups throughout the CNS (Wisden & Seeburg, 1993; Rodriguez-Moreno et al. 1997; Paarmann et al. 2000; Kieval et al. 2001). Understanding the contribution of KA receptors to glutamatergic signalling in the CNS, has lagged significantly behind AMPA (for review, see Palmer et al. 2005) and NMDA receptors (Hollmann & Heinemann, 1994), but development of agonists and antagonists specific to KA receptors has facilitated recent progress (Clarke et al. 1997; Bleakman et al. 2002; Gryder & Rogawski, 2003; More et al. 2004). GluR5 subunits in particular have been implicated in a variety of actions including regulation of synaptic inhibition (Clarke et al. 1997) and modulation of synaptic transmission and plasticity (Bortolotto et al. 1999; Sallert et al. 2007). Here we explored the role of this receptor subtype in both the generation of inspiratory rhythm and the transmission of the input to inspiratory motoneurons.
Inspiratory rhythm generation
This was not affected by UBP-302, whether used at concentrations specific for GluR5 subunit-containing KA receptors (More et al. 2004; Mayer et al. 2006) or at 10-fold greater concentrations when it interferes with AMPA receptor signalling. Note that the inability of bath-applied UBP-302 to alter inspiratory frequency was not due to inadequate drug diffusion. Local application of UBP-302 directly into the preBötC at a site where SP potently increased frequency (Gray et al. 1999; Guyenet & Wang, 2001) also failed to influence inspiratory rhythm. This suggests that KA receptors containing the GluR5 subunit do not have a significant role in the generation of inspiratory rhythm within the preBötC of the neonatal rat. Furthermore, since motor output from XII, C1 and C4 nerve roots is inhibited by UBP-302 at 100 but not 10 μm, differences in preBötC and motoneuron sensitivities probably reflect differential expression of AMPA receptors in the inspiratory synapses of these two network compartments.
Recent studies have suggested that in addition to the preBötC, a second more rostral site, the parafacial respiratory group (pFRG), also contributes to respiratory rhythm generation in mammals (Onimaru & Homma, 2003). Though the specific role of the pFRG is controversial, it is proposed to underlie the generation of active expiratory activity (Mellen et al. 2003; Janczewski & Feldman, 2006). At present little is known regarding the complement of Glu receptors in the pFRG nor their relative roles in the respiratory function of this cell group. We can say, however, that because UBP-302 was similarly without effect on inspiratory frequency in the brainstem–spinal cord preparation, which included the pFRG in our experiments, and the medullary slice, which did not contain the pFRG, that the contribution of the pFRG to respiratory rhythm generation in vitro does not involve GluR5 subunit-containing KA receptors.
XII motoneurons
These express functional GluR5 subunit-containing KA receptors that do not appear to contribute to inspiratory drive. The strongest evidence for functional GluR5 subunit-containing KA receptors is that in the absence or presence of TTX, the GluR5 agonist, ATPA, evoked in XII motoneurons inward currents that were slowly decaying and similar to those previously reported for dorsal root ganglion (DRG) neurons (which predominantly express GluR5) (Clarke et al. 1997), and GluR5 subunit-containing KA receptors in expression systems (Swanson et al. 1998). The affinity of ATPA for GluR5-expressing DRG neurons (Wong et al. 1994), GluR5 homomeric or GluR5/6, GluR5/KA2 heteromeric receptors is 100- to 1000-fold higher than for other Glu receptors, including GluR6 (Paternain et al. 1998) and KA2 (Clarke et al. 1997; Alt et al. 2004). The EC50 values for GluR5 range from 0.33 μm (see Table 1 in Alt et al. 2004) to 2.1 μm in HEK cells expressing human GluR5 (Clarke et al. 1997), while those for human GluR1, GlurR4 and GluR6 in HEK cells are >> 100 μm. Northern blot analyses also support the high affinity of ATPA for GluR5 subunits (Huettner, 1990; Partin et al. 1993; Bleakman et al. 1996), and that there is no specific binding of ATPA to cells expressing human GluR2 or GluR6 (Hoo et al. 1999).
The inhibition of ATPA currents by low concentrations (10 μm) of UBP-302 further supports the presence of functional GluR5 KA receptors on XII motoneurons (More et al. 2004; Mayer et al. 2006; Olsen et al. 2007). Thus, our data extend previous immunohistochemical evidence in mice (Paarmann et al. 2000) and rats (Garcia Del Cano et al. 1999) to demonstrate that XII motoneurons express functional GluR5 subunit-containing KA receptors.
Despite their presence, our data also suggest that GluR5 subunit-containing KA receptors are not involved in transmitting rhythmic inspiratory motor drive to XII, C1 or C4 motoneurons. Evidence is most compelling for C4 motor output where GluR5 subunit specific concentrations of UBP-302 (10 μm) (More et al. 2004; Mayer et al. 2006), had no effect on C4 inspiratory burst amplitude. A contribution of GluR5 subunit-containing KA receptors to the rhythmic inspiratory activity of C1 and XII motoneurons also appears unlikely. However, a minor role cannot be excluded. C1 and XII inspiratory burst amplitudes were 0.76 ± 0.08 (n = 4) and 0.82 ± 0.07 (n = 5) of control, respectively, in 10 μm UBP-302. These values were not statistically significant from control, but it remains possible that this reflects reduced power associated with low sample size.
It is also important to emphasize that these data do not rule out the possibility that GluR5-containing KA receptors might contribute to modulation of rhythm or motor output in more intact systems through endogenous pathways that are inactive in vitro. Both compartments (preBötC and motor nuclei) are sensitive to the exogenous activation of GluR5 receptors in vitro. In addition, similar in vitro–in vivo differences have been previously documented. For example, block of NMDA receptors has minimal effect on rhythmic inspiratory activity in neonates in vitro (Greer et al. 1991; Funk et al. 1993), but in more mature models in vivo it reduces inspiratory output more than 60%, reflecting a tonic excitatory NMDA receptor drive that is apparently absent in vitro, and upon which non-NMDA receptors mediate the majority of rhythmic drive (Krolo et al. 1999, 2000). Whether other subtypes of KA receptors contribute to the transmission of rhythmic inspiratory synaptic drive within rhythm-generating networks or motoneuron pools remains to be determined. Also of interest is whether the functional GluR5-containing KA receptors on XII motoneurons contribute to their non-respiratory functions such as chewing, mastication and swallowing.
Summary
In summary, we have established in neonatal rat in vitro that functional GluR5 subunit-containing KA receptors are likely to be on preBötC neurons and that they are present on XII motoneurons, but do not contribute to the generation or transmission of endogenous, rhythmic inspiratory activity. Most importantly, however, we provide the first functional evidence that AMPA receptors (or possibly non-GluR5-containing KA receptors) in rhythm-generating and motor output compartments of the respiratory network are not identical. AMPA receptors involved in the generation of inspiratory rhythm and the transmission of this inspiratory drive to motoneurons are differentially sensitive to UBP-302. Whether this persists developmentally remains to be established. However, it is the type of difference that may ultimately be exploited pharmacologically to enable the specific modulation of frequency, for example to counteract drug-induced central apnoeas as well as those associated with disease and apnoea of prematurity, or respiratory motor output (including airway muscle activity) during wake–sleep transitions to counteract obstructive forms of sleep-disordered breathing.
Acknowledgments
This work was supported by the Alberta Heritage Foundation for Medical Research (AHFMR), Canadian Institutes for Health Research (CIHR), Canadian Foundation for Innovation (CFI), Alberta Science and Research Authority (ASRA), Health Research Council of New Zealand (HRC) and Auckland Medical Research Foundation (AMRF). A.R.L. was supported by studentships from HRC and AMRF and is currently a postdoctoral fellow supported by AHFMR. M.F.I., A.R.L., F.C.L., T.A. and T.S.A. were all supported by the Strategic Training Program in Maternal-Fetal-Newborn Health (MFN Health) of the Institute for Human Development, Child and Youth Health, Canadian Institutes of Health Research (CIHR-IHDCYH). J.J.G. and G.D.F. are Scientist and Senior Scholar of the AHFMR, respectively.
References
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, Large TH, Bleakman D. Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology. 2004;46:793–806. doi: 10.1016/j.neuropharm.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Gates MR, Ogden AM, MacKowiak M. Kainate receptor agonists, antagonists and allosteric modulators. Curr Pharm Des. 2002;8:873–885. doi: 10.2174/1381612024607108. [DOI] [PubMed] [Google Scholar]
- Bleakman R, Schoepp DD, Ballyk B, Bufton H, Sharpe EF, Thomas K, Ornstein PL, Kamboj RK. Pharmacological discrimination of GluR5 and GluR6 kainate receptor subtypes by (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahydroisdoquinoline-3 carboxylic acid. Mol Pharmacol. 1996;49:581–585. [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Johnson SM, Smith JC, Dong XW, Lai J, Feldman JL. Functional respiratory rhythm generating networks in neonatal mice lacking NMDAR1 gene. J Neurophysiol. 1997;78:1414–1420. doi: 10.1152/jn.1997.78.3.1414. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Modulation of neural network activity in vitro by cyclothiazide, a drug that blocks desensitization of AMPA receptors. J Neurosci. 1995;15:4046–4056. doi: 10.1523/JNEUROSCI.15-05-04046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Del Cano G, Millan LM, Gerrikagoitia I, Sarasa M, Matute C. Ionotropic glutamate receptor subunit distribution on hypoglossal motoneuronal pools in the rat. J Neurocytol. 1999;28:455–468. doi: 10.1023/a:1007001020700. [DOI] [PubMed] [Google Scholar]
- Ge Q, Feldman JL. AMPA receptor activation and phosphatase inhibition affect neonatal rat respiratory rhythm generation. J Physiol. 1998;509:255–266. doi: 10.1111/j.1469-7793.1998.255bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryder DS, Rogawski MA. Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J Neurosci. 2003;23:7069–7074. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Wang H. Pre-Bötzinger neurons with preinspiratory discharges ‘in vivo’ express NK1 receptors in the rat. J Neurophysiol. 2001;86:438–446. doi: 10.1152/jn.2001.86.1.438. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hoo K, Legutko B, Rizkalla G, Deverill M, Hawes CR, Ellis GJ, Stensbol TB, Krogsgaard-Larsen P, Skolnick P, Bleakman D. [3H]ATPA: a high affinity ligand for GluR5 kainate receptors. Neuropharmacology. 1999;38:1811–1817. doi: 10.1016/s0028-3908(99)00133-1. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Ireland MF, Noakes PG, Bellingham MC. P2×7-like receptor subunits enhance excitatory synaptic transmission at central synapses by presynaptic mechanisms. Neuroscience. 2004;128:269–280. doi: 10.1016/j.neuroscience.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieval JZ, Hubert GW, Charara A, Pare JF, Smith Y. Subcellular and subsynaptic localization of presynaptic and postsynaptic kainate receptor subunits in the monkey striatum. J Neurosci. 2001;21:8746–8757. doi: 10.1523/JNEUROSCI.21-22-08746.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Sakai A. A study on the localization of the sternocleidomastoid and trapezius motoneurons in the rat by means of the HRP method. Anat Rec. 1982;202:527–536. doi: 10.1002/ar.1092020412. [DOI] [PubMed] [Google Scholar]
- Krolo M, Stuth EA, Tonkovic-Capin M, Dogas Z, Hopp FA, Mccrimmon DR, Zuperku EJ. Differential roles of ionotropic glutamate receptors in canine medullary inspiratory neurons of the ventral respiratory group. J Neurophysiol. 1999;82:60–68. doi: 10.1152/jn.1999.82.1.60. [DOI] [PubMed] [Google Scholar]
- Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, Mccrimmon DR, Zuperku EJ. Relative magnitude of tonic and phasic synaptic excitation of medullary inspiratory neurons in dogs. Am J Physiol Regulatory Integrative Comp Physiol. 2000;279:R639–R649. doi: 10.1152/ajpregu.2000.279.2.R639. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Presynaptic kainate receptors in the hippocampus: slowly emerging from obscurity. Neuron. 2001;32:561–564. doi: 10.1016/s0896-6273(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999;397:161–164. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Greer JJ, Feldman JL. Phrenic motoneuron morphology in the neonatal rat. J Comp Neurol. 1991;308:169–179. doi: 10.1002/cne.903080204. [DOI] [PubMed] [Google Scholar]
- Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci U S A. 2004;101:3172–3177. doi: 10.1073/pnas.0306731101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorier AR, Huxtable AG, Robinson DM, Lipski J, Housley GD, Funk GD. P2Y1 receptor modulation of the pre-Bötzinger complex inspiratory rhythm generating network in vitro. J Neurosci. 2007;27:993–1005. doi: 10.1523/JNEUROSCI.3948-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Smith JC, Feldman JL. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. J Neurosci. 1989;9:1910–1921. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Ghosal A, Dolman NP, Jane DE. Crystal structures of the kainate receptor GluR5 ligand binding core dimer with novel GluR5-selective antagonists. J Neurosci. 2006;26:2852–2861. doi: 10.1523/JNEUROSCI.0123-06.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Parkis MA, Lipski J, Funk GD. Modulation of phrenic motoneuron excitability by ATP: consequences for respiratory-related output in vitro. J Appl Physiol. 2002;92:1899–1910. doi: 10.1152/japplphysiol.00475.2001. [DOI] [PubMed] [Google Scholar]
- More JC, Nistico R, Dolman NP, Clarke VR, Alt AJ, Ogden AM, Buelens FP, Troop HM, Kelland EE, Pilato F, Bleakman D, Bortolotto ZA, Collingridge GL, Jane DE. Characterisation of UBP296: a novel, potent and selective kainate receptor antagonist. Neuropharmacology. 2004;47:46–64. doi: 10.1016/j.neuropharm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Olsen DP, Dunlap K, Jacob MH. Kainate receptors and RNA editing in cholinergic neurons. J Neurochem. 2007;101:327–341. doi: 10.1111/j.1471-4159.2006.04359.x. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paarmann I, Frermann D, Keller BU, Hollmann M. Expression of 15 glutamate receptor subunits and various splice variants in tissue slices and single neurons of brainstem nuclei and potential functional implications. J Neurochem. 2000;74:1335–1345. doi: 10.1046/j.1471-4159.2000.0741335.x. [DOI] [PubMed] [Google Scholar]
- Pace RW, MacKay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Adachi T, Ren J, Funk GD, Greer JJ. Fluorescent tagging of rhythmically active respiratory neurons within the pre-Bötzinger complex of rat medullary slice preparations. J Neurosci. 2005;25:2591–2596. doi: 10.1523/JNEUROSCI.4930-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CL, Cotton L, Henley JM. The molecular pharmacology and cell biology of a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacol Rev. 2005;57:253–277. doi: 10.1124/pr.57.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Rodriguez-Moreno A, Villarroel A, Lerma J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology. 1998;37:1249–1259. doi: 10.1016/s0028-3908(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML, Jane DE, Watkins JC. Activation and desensitization of AMPA/kainate receptors by novel derivatives of willardiine. J Neurosci. 1992;12:595–606. doi: 10.1523/JNEUROSCI.12-02-00595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JC, Hesson DP, Jones KA, Costa AM. Substituted 1,2-dihydrophthalazines: potent, selective and non-competitive inhibitors of the AMPA receptor. J Medicalchem. 1996;39:343–346. doi: 10.1021/jm950740w. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Robinson D, Ellenberger H. Distribution of N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptor subunits on respiratory motor and premotor neurons in the rat. J Comp Neurol. 1997;389:94–116. doi: 10.1002/(sici)1096-9861(19971208)389:1<94::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallert M, Malkki H, Segerstrale M, Taira T, Lauri SE. Effects of the kainate receptor agonist ATPA on glutamatergic synaptic transmission and plasticity during early postnatal development. Neuropharmacology. 2007;52:1354–1365. doi: 10.1016/j.neuropharm.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Green T, Heinemann SF. Kainate receptors exhibit differential sensitivities to (S)-5-iodowillardiine. Mol Pharmacol. 1998;53:942–949. [PubMed] [Google Scholar]
- Ullah M, Mansor O, Ismail ZI, Kapitonova MY, Sirajudeen KN. Localization of the spinal nucleus of the accessory nerve in rat: a horseradish peroxidase study. J Anat. 2007;210:428–438. doi: 10.1111/j.1469-7580.2007.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Calcium conductances and their role in the firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol. 1993;69:2137–2149. doi: 10.1152/jn.1993.69.6.2137. [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LA, Mayer ML, Jane DE, Watkins JC. Willardiines differentiate agonist binding sites for kainate- versus AMPA-preferring glutamate receptors in DRG and hippocampal neurons. J Neurosci. 1994;14:3881–3897. doi: 10.1523/JNEUROSCI.14-06-03881.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman S, Lodge D. Pharmacological characterization of non-NMDA subtypes of glutamate receptor in the neonatal rat hemisected spinal cord in vitro. Br J Pharmacol. 1992;106:367–372. doi: 10.1111/j.1476-5381.1992.tb14342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


