Abstract
The muscle pump and muscle vasodilatory mechanims are thought to play important roles in increasing and maintaining muscle perfusion and cardiac output 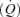 during exercise, but their actual contributions remain uncertain. To evaluate the role of the skeletal muscle pump and vasodilatation on cardiovascular function during exercise, we determined leg and systemic haemodynamic responses in healthy men during (1) incremental one-legged knee-extensor exercise, (2) step-wise femoral artery ATP infusion at rest, (3) passive exercise (n = 10), (4) femoral vein or artery ATP infusion (n = 6), and (5) cyclic thigh compressions at rest and during passive and voluntary exercise (n = 7). Incremental exercise resulted in progressive increases in leg blood flow (ΔLBF 7.4 ± 0.7 l min−1), cardiac output (
during exercise, but their actual contributions remain uncertain. To evaluate the role of the skeletal muscle pump and vasodilatation on cardiovascular function during exercise, we determined leg and systemic haemodynamic responses in healthy men during (1) incremental one-legged knee-extensor exercise, (2) step-wise femoral artery ATP infusion at rest, (3) passive exercise (n = 10), (4) femoral vein or artery ATP infusion (n = 6), and (5) cyclic thigh compressions at rest and during passive and voluntary exercise (n = 7). Incremental exercise resulted in progressive increases in leg blood flow (ΔLBF 7.4 ± 0.7 l min−1), cardiac output (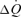 8.7 ± 0.7 l min−1), mean arterial pressure (ΔMAP 51 ± 5 mmHg), and leg and systemic oxygen delivery and
8.7 ± 0.7 l min−1), mean arterial pressure (ΔMAP 51 ± 5 mmHg), and leg and systemic oxygen delivery and 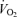 . Arterial ATP infusion resulted in similar increases in
. Arterial ATP infusion resulted in similar increases in 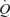 , LBF, and systemic and leg oxygen delivery, but central venous pressure and muscle metabolism remained unchanged and MAP was reduced. In contrast, femoral vein ATP infusion did not alter LBF,
, LBF, and systemic and leg oxygen delivery, but central venous pressure and muscle metabolism remained unchanged and MAP was reduced. In contrast, femoral vein ATP infusion did not alter LBF, 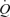 or MAP. Passive exercise also increased blood flow (ΔLBF 0.7 ± 0.1 l min−1), yet the increase in muscle and systemic perfusion, unrelated to elevations in aerobic metabolism, accounted only for ∼5% of peak exercise hyperaemia. Likewise, thigh compressions alone or in combination with passive exercise increased blood flow (ΔLBF 0.5–0.7 l min−1) without altering
or MAP. Passive exercise also increased blood flow (ΔLBF 0.7 ± 0.1 l min−1), yet the increase in muscle and systemic perfusion, unrelated to elevations in aerobic metabolism, accounted only for ∼5% of peak exercise hyperaemia. Likewise, thigh compressions alone or in combination with passive exercise increased blood flow (ΔLBF 0.5–0.7 l min−1) without altering 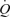 , MAP or
, MAP or 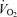 . These findings suggest that the skeletal muscle pump is not obligatory for sustaining venous return, central venous pressure, stroke volume and
. These findings suggest that the skeletal muscle pump is not obligatory for sustaining venous return, central venous pressure, stroke volume and 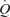 or maintaining muscle blood flow during one-legged exercise in humans. Further, its contribution to muscle and systemic peak exercise hyperaemia appears to be minimal in comparison to the effects of muscle vasodilatation.
or maintaining muscle blood flow during one-legged exercise in humans. Further, its contribution to muscle and systemic peak exercise hyperaemia appears to be minimal in comparison to the effects of muscle vasodilatation.
During exercise, blood flow to contracting skeletal muscles increases to ensure supply of oxygen and substrates and enhance removal of metabolic by-products. This exercise-induced elevation in muscle perfusion takes place in conjunction with an increase in cardiac output  , brought about by an increase in both stroke volume (SV) and heart rate (HR; Higginbotham et al. 1986). The mechanisms controlling local and systemic perfusion are thought to implicate mechanical, neurological and metabolic factors (Shepherd, 1983; Laughlin et al. 1996; Rowell, 2004; Saltin, 2007). Conventionally, the skeletal muscle pump (i.e. the lumped functions including local and central circulatory effects (Sheriff, 2005)) is deemed to be vital in coordinating the local and systemic blood flow responses by enhancing venous return, central venous pressure (CVP), end-diastolic volume of the heart and thus SV and
, brought about by an increase in both stroke volume (SV) and heart rate (HR; Higginbotham et al. 1986). The mechanisms controlling local and systemic perfusion are thought to implicate mechanical, neurological and metabolic factors (Shepherd, 1983; Laughlin et al. 1996; Rowell, 2004; Saltin, 2007). Conventionally, the skeletal muscle pump (i.e. the lumped functions including local and central circulatory effects (Sheriff, 2005)) is deemed to be vital in coordinating the local and systemic blood flow responses by enhancing venous return, central venous pressure (CVP), end-diastolic volume of the heart and thus SV and 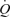 during exercise (Rowell et al. 1986; Notarius & Magder, 1996). Further, the muscle pump is thought to play an important role in muscle hyperaemia from the onset of exercise, although its significance remains contentious with some reports supporting (Pollack & Wood, 1949; Folkow et al. 1971; Shiotani et al. 2002) and other refuting (Laughlin & Schrage, 1999; Dodson & Gladden, 2003; Hamann et al. 2003) a significant contribution (for review, Laughlin, 1987; Laughlin & Joyner, 2003).
during exercise (Rowell et al. 1986; Notarius & Magder, 1996). Further, the muscle pump is thought to play an important role in muscle hyperaemia from the onset of exercise, although its significance remains contentious with some reports supporting (Pollack & Wood, 1949; Folkow et al. 1971; Shiotani et al. 2002) and other refuting (Laughlin & Schrage, 1999; Dodson & Gladden, 2003; Hamann et al. 2003) a significant contribution (for review, Laughlin, 1987; Laughlin & Joyner, 2003).
The discrepancy between blood flows achieved by maximal pharmacological vasodilatation and exercise is one piece of evidence supporting the muscle pump hypothesis (Rowell, 2004; Sheriff, 2005). Studies in upright seated healthy humans, however, show that femoral artery infusion of ATP or adenosine increase leg blood flow (LBF) to levels similar to peak exercise hyperaemia (i.e. 7–8 l min−1) (Rådegran & Calbet, 2001; González-Alonso et al. 2002; Rosenmeier et al. 2004), but their effects on central cardiovascular variables are unknown. According to the muscle pump hypothesis, pharmacologically induced muscle hyperaemia would be limited at least partially by insufficient venous return, CVP, SV and thus 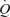 as the skeletal muscle pump is not assisting venous return from the lower extremities to the heart (Rowell et al. 1996). To date, no study has compared leg vasodilatation to exercise hyperaemia in humans to determine whether or not the muscle pump is necessary for the maintenance of central haemodynamic function.
as the skeletal muscle pump is not assisting venous return from the lower extremities to the heart (Rowell et al. 1996). To date, no study has compared leg vasodilatation to exercise hyperaemia in humans to determine whether or not the muscle pump is necessary for the maintenance of central haemodynamic function.
Partition of the muscle pump from other mechanical and metabolic effects of muscle contraction on blood flow is crucial to determine its independent contribution to exercise hyperaemia. Studies of humans using passive and voluntary knee-extensor exercise in the upright and supine positions (Wray et al. 2005) and external whole forearm compressions (Kirby et al. 2007) indicate a contribution of mechanically induced vasodilatation to onset exercise hyperaemia. These studies, however, did not simultaneously measure muscle metabolism to ensure that the proposed influences of the muscle pump and mechanical deformation on perfusion are independent of metabolic mechanisms. Supporting an independent mechanically driven vasodilatory pathway, mechanical deformation of rat skeletal muscle feed arteries in vitro via increases in extravascular pressure induces rapid vasodilatation (Clifford et al. 2006). Whether increases in extravascular pressure in the human thigh lead to muscle vasodilatation and elevations in  beyond what is found during passive and dynamic knee-extensor exercise has not been investigated.
beyond what is found during passive and dynamic knee-extensor exercise has not been investigated.
The principal aim of this study was to determine the influence of leg vasodilatation on central cardiovascular responses and, specifically, on CVP, SV and 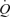 and compare these responses with those observed during exercise. We hypothesized that arterial ATP infusion increases SV and
and compare these responses with those observed during exercise. We hypothesized that arterial ATP infusion increases SV and 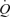 to levels seen during exercise without the activation of the muscle pump. A second aim of the study was to examine the contribution of the skeletal muscle pump and mechanical deformation on local and systemic exercise hyperaemia by comparing haemodynamic responses during passive and active knee-extensor exercise, cyclic thigh compressions alone or in combination with passive and voluntary exercise and separate femoral vein and artery ATP infusion. We hypothesized that the contribution of the skeletal muscle pump and mechanical vasodilatatory mechanisms to local and systemic peak exercise hyperaemia is small in comparison to the effects of muscle vasodilatation.
to levels seen during exercise without the activation of the muscle pump. A second aim of the study was to examine the contribution of the skeletal muscle pump and mechanical deformation on local and systemic exercise hyperaemia by comparing haemodynamic responses during passive and active knee-extensor exercise, cyclic thigh compressions alone or in combination with passive and voluntary exercise and separate femoral vein and artery ATP infusion. We hypothesized that the contribution of the skeletal muscle pump and mechanical vasodilatatory mechanisms to local and systemic peak exercise hyperaemia is small in comparison to the effects of muscle vasodilatation.
Methods
Twenty-three healthy subjects participated in three studies. Ten recreationally active males participated in the first study (Study 1). They had a mean (±s.d.) age of 26 ± 3 years, body weight of 83 ± 11 kg, height of 189 ± 8 cm and a maximal oxygen uptake 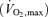 of 4.8 ± 0.4 l min−1. In Study 2, the subjects were three healthy males and three females aged 39 ± 3 years, weight 67 ± 4 kg, height 171 ± 2 cm and a
of 4.8 ± 0.4 l min−1. In Study 2, the subjects were three healthy males and three females aged 39 ± 3 years, weight 67 ± 4 kg, height 171 ± 2 cm and a 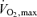 of 2.8 ± 0.1 l min−1. In Study 3, the subjects were seven recreationally active males aged 27 ± 5 years, weight 81 ± 9 kg and height 181 ± 7 cm. The subjects were fully informed of any risks and discomforts associated with the experiments before providing their informed written consent to participate. The studies conformed to the code of Ethics of the World Medical Association (Declaration of Helsinki) and were approved by the Research Ethics Committees of Copenhagen and Frederiksberg communities (Studies 1 and 2) and Brunel University (Study 3).
of 2.8 ± 0.1 l min−1. In Study 3, the subjects were seven recreationally active males aged 27 ± 5 years, weight 81 ± 9 kg and height 181 ± 7 cm. The subjects were fully informed of any risks and discomforts associated with the experiments before providing their informed written consent to participate. The studies conformed to the code of Ethics of the World Medical Association (Declaration of Helsinki) and were approved by the Research Ethics Committees of Copenhagen and Frederiksberg communities (Studies 1 and 2) and Brunel University (Study 3).
Experimental protocols
In Study 1, leg and systemic haemodynamic responses were examined during both incremental femoral artery infusions of ATP (1, 4, 16 and 64 μmol min−1) and incremental one-legged knee-extensor exercise to 93 ± 3% of peak power (26 ± 3, 52 ± 6, 79 ± 9 and 95 ± 11 W). ATP (Sigma A7699) was dissolved in isotonic saline and infused into the femoral artery during stepwise 1.5 min periods. Each exercise stage lasted 1.5 min. In addition, leg and systemic haemodynamics were examined during passive leg-kicking exercise. All protocols were separated by 30 min of recovery. In Study 2, leg and systemic haemodynamics were examined (1) at rest, (2) during and after ATP infusion in the femoral artery or vein (1 and 15 μmol min−1), and (3) during and after recovery from peak knee-extensor exercise (53 ± 7 W; ∼60 r.p.m.). In Study 3, leg and systemic haemodynamics were examined with and without thigh compressions (at a rate of 60 Hz): (1) at rest, (2) during passive leg-kicking exercise, and (3) during light one-legged knee-extensor exercise (20 W; ∼20% of peak power). In Studies 1 and 2, the subjects were examined in the upright-seated position and in Study 3 in the semirecumbent position. The haemodynamic effects of cyclic thigh compressions were also examined when subjects were in the upright-seated and the supine positions.
Instrumentation of subjects
On the first visit to the laboratory of Study 1, the subjects performed incremental knee-extensor and cycle ergometer exercise (Excalibur, Lode, the Netherlands) to determine peak power and 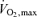 . On the day of the experiment, the subjects reported to the laboratory at 8 a.m. following ingestion of their usual breakfast. Upon arrival, subjects rested in the supine position while catheters were placed under local anaesthesia into the femoral artery and vein of the exercising thigh and the brachial artery and an antecubital vein, with the latter catheter being advanced to the right atrium. The femoral artery and vein catheters were positioned 1–2 cm proximal or distal from the inguinal ligament. A thermistor to measure venous blood temperature was inserted through the femoral catheter orientated in the anterograde direction for the determination of femoral blood flow. The subjects then walked to the experimental room and sat on the knee-extensor ergometer while instrumentation was attached. Following ∼10 min of seated rest, baseline leg and systemic haemodynamics and blood samples were obtained. During each stage of either ATP infusion or incremental knee-extensor exercise, arterial and femoral venous blood samples were withdrawn after 40 s followed by the measurement of LBF at 60 s. Measurements were also obtained after 10 min of recovery. During the passive-exercise protocol, the subject's leg strapped to the ergometer was moved passively with the same frequency and range of motion as during dynamic knee-extensor exercise. In Study 2, catheters for blood sampling and ATP infusion were inserted in the femoral artery and vein whereas in Study 3 they were inserted in the radial artery and femoral vein following the procedures described for Study 1. Active exercise in the subsequent studies was performed as described above. Passive exercise, however, was performed with the leg free from the ergometer to minimize muscle metabolic activity. In all studies, the lower legs were below heart level.
. On the day of the experiment, the subjects reported to the laboratory at 8 a.m. following ingestion of their usual breakfast. Upon arrival, subjects rested in the supine position while catheters were placed under local anaesthesia into the femoral artery and vein of the exercising thigh and the brachial artery and an antecubital vein, with the latter catheter being advanced to the right atrium. The femoral artery and vein catheters were positioned 1–2 cm proximal or distal from the inguinal ligament. A thermistor to measure venous blood temperature was inserted through the femoral catheter orientated in the anterograde direction for the determination of femoral blood flow. The subjects then walked to the experimental room and sat on the knee-extensor ergometer while instrumentation was attached. Following ∼10 min of seated rest, baseline leg and systemic haemodynamics and blood samples were obtained. During each stage of either ATP infusion or incremental knee-extensor exercise, arterial and femoral venous blood samples were withdrawn after 40 s followed by the measurement of LBF at 60 s. Measurements were also obtained after 10 min of recovery. During the passive-exercise protocol, the subject's leg strapped to the ergometer was moved passively with the same frequency and range of motion as during dynamic knee-extensor exercise. In Study 2, catheters for blood sampling and ATP infusion were inserted in the femoral artery and vein whereas in Study 3 they were inserted in the radial artery and femoral vein following the procedures described for Study 1. Active exercise in the subsequent studies was performed as described above. Passive exercise, however, was performed with the leg free from the ergometer to minimize muscle metabolic activity. In all studies, the lower legs were below heart level.
Thigh compressions
In Study 3, increases in extravascular pressure were induced using two custom-made blood pressure cuffs that were wrapped around the thigh and rapidly inflated and deflated with a cuff inflation unit (Hokanson E20, Bellevue, WA, USA). To simulate knee-extensor exercise closely, the cuff was inflated to 200 mmHg at a rate of 60 Hz, which is the same frequency as during exercise. This was the highest pressure-to-frequency ratio that could be produced. During exercise, cuff inflation and force development were synchronized using a triggering system (PowerLab 16/30, ADInstruments, Bella Vista, NSW 2153, Australia), such that the cuff inflation was superimposed upon quadriceps muscle contraction and deflation occurred during relaxation. During dynamic knee-extensor exercise, the relaxation phase is passive as the leg is moved backwards by the ergometer's motor.
Leg and systemic haemodynamics and oxygenation
Throughout all studies, pulmonary 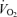 was measured online (Cosmed Quark b 2, Italy), HR was obtained from an electrocardiogram while vascular pressures were measured at heart level in artery and right atrium or femoral vein with transducers (Pressure Monitoring Kit, Baxter) connected to a monitor (Dialogue 2000, Danica Electronic, Copenhagen, Denmark) (Studies 1 and 2) or BP amplifiers and a data acquisition system (Power Laboratory 16/30, ADInstruments; Study 3). The LBF was measured by the constant-infusion thermodilution method in Study 1 (Andersen & Saltin, 1985; González-Alonso et al. 2000) and by ultrasound Doppler in Studies 2 and 3 (GE Logiq 5 and GE Vivid 7, General Electrics, USA) (Rådegran, 1997). In all studies,
was measured online (Cosmed Quark b 2, Italy), HR was obtained from an electrocardiogram while vascular pressures were measured at heart level in artery and right atrium or femoral vein with transducers (Pressure Monitoring Kit, Baxter) connected to a monitor (Dialogue 2000, Danica Electronic, Copenhagen, Denmark) (Studies 1 and 2) or BP amplifiers and a data acquisition system (Power Laboratory 16/30, ADInstruments; Study 3). The LBF was measured by the constant-infusion thermodilution method in Study 1 (Andersen & Saltin, 1985; González-Alonso et al. 2000) and by ultrasound Doppler in Studies 2 and 3 (GE Logiq 5 and GE Vivid 7, General Electrics, USA) (Rådegran, 1997). In all studies, 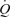 was calculated by multiplying SV by HR, using the modelflow method to determine SV (Beat Scope 1.1a; Finapres Medical Systems BV, Amsterdam, the Netherlands) (Bogert & van Lieshout, 2005; González-Alonso et al. 2006). Systemic and leg vascular conductance was calculated as the ratio between
was calculated by multiplying SV by HR, using the modelflow method to determine SV (Beat Scope 1.1a; Finapres Medical Systems BV, Amsterdam, the Netherlands) (Bogert & van Lieshout, 2005; González-Alonso et al. 2006). Systemic and leg vascular conductance was calculated as the ratio between 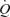 or LBF and MAP. For systemic O2 delivery,
or LBF and MAP. For systemic O2 delivery,  was multiplied by the arterial O2 content whereas systemic O2 extraction was the ratio between the systemic arterial–venous (a–v) O2 difference and the arterial O2 content. Blood gas variables and haemoglobin concentrations were measured using ABL700 or ABL 825 analysers (Radiometer, Copenhagen, Denmark).
was multiplied by the arterial O2 content whereas systemic O2 extraction was the ratio between the systemic arterial–venous (a–v) O2 difference and the arterial O2 content. Blood gas variables and haemoglobin concentrations were measured using ABL700 or ABL 825 analysers (Radiometer, Copenhagen, Denmark).
Statistical analysis
One- or two-way repeated measure of variance (ANOVA) was performed to test significant differences between treatments. Following a significant F test, pair-wise differences were identified using Tukey's post hoc test. The significant level was set at P < 0.05 and data are presented as mean ±s.e.m.
Results
Incremental knee-extensor exercise and arterial ATP infusion
During incremental one-legged knee-extensor exercise, 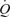 , LBF, MAP and systemic and leg O2 delivery and
, LBF, MAP and systemic and leg O2 delivery and 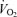 increased progressively and returned to baseline values after 10 min of recovery (Fig. 1). The rate of increase in LBF and leg
increased progressively and returned to baseline values after 10 min of recovery (Fig. 1). The rate of increase in LBF and leg 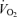 was 79 ± 8 and 13 ± 1 ml min−1 W−1, respectively (r2= 0.99; P < 0.001) and the increase in LBF and
was 79 ± 8 and 13 ± 1 ml min−1 W−1, respectively (r2= 0.99; P < 0.001) and the increase in LBF and 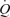 was 6.1 ± 0.3 and 5.5 ± 0.4 l min−1
was 6.1 ± 0.3 and 5.5 ± 0.4 l min−1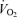 −1, respectively (P= 0.73; r2= 0.99; P < 0.001). The increase in
−1, respectively (P= 0.73; r2= 0.99; P < 0.001). The increase in 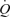 above ∼30% peak power was associated with a plateau in SV (P = 0.15 from 30% to 93% peak power) and an exponential rise in HR. The increase in LBF reflected an increase in leg vascular conductance and perfusion pressure.
above ∼30% peak power was associated with a plateau in SV (P = 0.15 from 30% to 93% peak power) and an exponential rise in HR. The increase in LBF reflected an increase in leg vascular conductance and perfusion pressure.
Figure 1. Leg and systemic haemodynamics during incremental knee-extensor exercise.
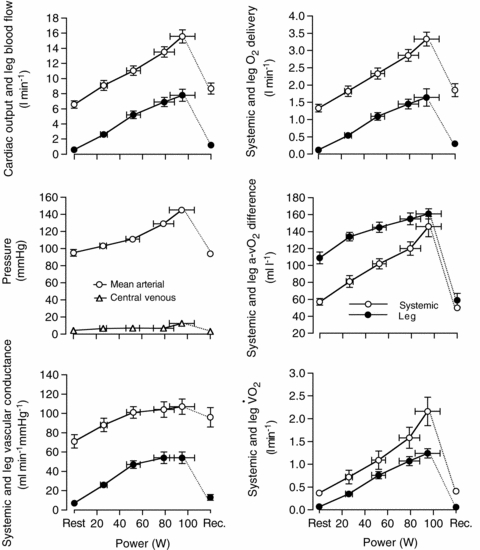
Cardiovascular variables plotted against increases in power output during one-legged knee-extensor exercise. Data are mean ±s.e.m. for 9–10 subjects.
Graded femoral artery infusion of ATP evoked significant increases in 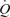 , LBF, and leg and systemic O2 delivery, even though MAP, CVP and perfusion pressure remained stable. When plotted against the increase in LBF, the rise in
, LBF, and leg and systemic O2 delivery, even though MAP, CVP and perfusion pressure remained stable. When plotted against the increase in LBF, the rise in 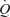 and O2 delivery were not different during ATP infusion and exercise (Fig. 2). However, in contrast to the plateau observed during exercise, arterial ATP infusion resulted in a linear increase in SV accompanying a lower rate of rise in HR. Therefore, SV at LBF > 5 l min−1 and
and O2 delivery were not different during ATP infusion and exercise (Fig. 2). However, in contrast to the plateau observed during exercise, arterial ATP infusion resulted in a linear increase in SV accompanying a lower rate of rise in HR. Therefore, SV at LBF > 5 l min−1 and 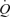 > 11 l min−1 was higher during ATP infusion compared with exercise even though CVP was lower at peak leg perfusion. Also, in contrast to exercise, arterial ATP infusion did not change metabolism across the leg, as indicated by the unchanged leg
> 11 l min−1 was higher during ATP infusion compared with exercise even though CVP was lower at peak leg perfusion. Also, in contrast to exercise, arterial ATP infusion did not change metabolism across the leg, as indicated by the unchanged leg 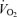 , glucose uptake, lactate release and the declining femoral venous temperature (Table 1). Blood flow to non-exercising or non-infused leg tissues (i.e.
, glucose uptake, lactate release and the declining femoral venous temperature (Table 1). Blood flow to non-exercising or non-infused leg tissues (i.e. 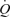 – LBF) remained at ∼6 l min−1 during mild and moderate exercise and mild and moderate arterial ATP infusion, but tended to increase at peak exercise and ATP-induced hyperaemia (6.6–7.6 l min−1). Lastly, both leg and systemic vascular conductance were elevated at LBF > 7 l min−1 during ATP infusion compared to exercise. The elevated systemic vascular conductance at the two highest levels of hyperaemia during arterial ATP infusion compared to exercise was associated with higher vascular conductance in both the leg and the rest of the body tissues, with the former accounting for ∼60% of the systemic difference.
– LBF) remained at ∼6 l min−1 during mild and moderate exercise and mild and moderate arterial ATP infusion, but tended to increase at peak exercise and ATP-induced hyperaemia (6.6–7.6 l min−1). Lastly, both leg and systemic vascular conductance were elevated at LBF > 7 l min−1 during ATP infusion compared to exercise. The elevated systemic vascular conductance at the two highest levels of hyperaemia during arterial ATP infusion compared to exercise was associated with higher vascular conductance in both the leg and the rest of the body tissues, with the former accounting for ∼60% of the systemic difference.
Figure 2. Leg and systemic haemodynamics with incremental exercise and graded intrafemoral artery ATP infusion.
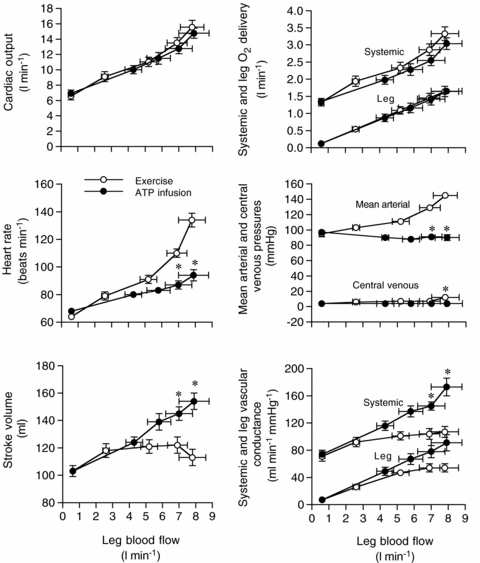
Cardiovascular variables plotted against leg blood flow during incremental one-legged knee-extensor exercise and graded intrafemoral artery ATP infusion. Open circles depict systemic haemodynamics whereas filled circles depict leg haemodynamics. Data are mean ±s.e.m. for 9 subjects. *Different from exercise, P < 0.05.
Table 1.
Femoral blood variables during one-legged knee-extensor exercise and femoral artery ATP infusion
| Conditions | Knee-extensor exercise (W) | Intrafemoral artery ATP infusion (μmol min−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 26 ± 3 | 52 ± 6 | 79 ± 9 | 95 ± 11 | Recovery | Baseline | 1 | 4 | 16 | 64 | Recovery | |
| Haemoglobin (g l−1) | ||||||||||||
| a | 150 ± 2 | 154 ± 4 | 155 ± 4 | 156 ± 4 | 157 ± 4 | 156 ± 4 | 145 ± 2 | 146 ± 3 | 146 ± 2 | 147 ± 3 | 149 ± 2 | 147 ± 2 |
| v | 149 ± 2 | 152 ± 2 | 156 ± 3 | 158 ± 3 | 159 ± 4 | 158 ± 4 | 148 ± 2 | 149 ± 2 | 147 ± 2 | 148 ± 2 | 148 ± 8 | 147 ± 2 |
| O2 saturation (%) | ||||||||||||
| a | 98.2 ± 0.2 | 98.3 ± 0.2 | 98.3 ± 0.1 | 98.6 ± 0.1 | 98.7 ± 0.1 | 98.1 ± 0.3 | 98.3 ± 0.3 | 98.8 ± 0.2 | 99.0 ± 0.1 | 99.0 ± 0.1 | 99.4 ± 0.1 | 98.2 ± 0.2 |
| v | 45.1 ± 3.6 | 35.2 ± 1.8 | 29.7 ± 1.6 | 25.1 ± 1.7 | 23.4 ± 1.5 | 69.5 ± 4.3 | 53.6 ± 4.5 | 93.6 ± 1.1* | 96.5 ± 0.3* | 96.8 ± 0.5* | 97.1 ± 0.2* | 64.0 ± 5.2 |
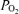 (mmHg) (mmHg) | ||||||||||||
| a | 100 ± 3 | 103 ± 3 | 103 ± 2 | 111 ± 2 | 114 ± 3 | 105 ± 3 | 100 ± 4 | 109 ± 4 | 113 ± 3 | 112 ± 3 | 122 ± 2* | 102 ± 2 |
| v | 25 ± 1 | 22 ± 1 | 21 ± 1 | 21 ± 1 | 21 ± 1 | 41 ± 3 | 29 ± 2 | 69 ± 3* | 79 ± 2* | 79 ± 2* | 81 ± 2* | 35 ± 4 |
| O2 content (ml l−1) | ||||||||||||
| a | 200 ± 3 | 206 ± 5 | 207 ± 5 | 209 ± 5 | 212 ± 5 | 209 ± 5 | 194 ± 3 | 196 ± 4 | 197 ± 3 | 198 ± 4 | 202 ± 5 | 197 ± 3 |
| v | 91 ± 8 | 72 ± 4 | 62 ± 3 | 54 ± 3 | 50 ± 3 | 149 ± 12 | 107 ± 9* | 189 ± 4* | 192 ± 3* | 195 ± 3* | 195 ± 3* | 128 ± 1* |
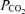 (mmHg) (mmHg) | ||||||||||||
| a | 39 ± 1 | 40 ± 1 | 41 ± 1 | 40 ± 1 | 37 ± 1 | 37 ± 1 | 36 ± 1 | 37 ± 1* | 36 ± 1* | 35 ± 1* | 33 ± 1* | 38 ± 1 |
| v | 45 ± 1 | 52 ± 1 | 59 ± 2 | 71 ± 2 | 79 ± 3 | 43 ± 1 | 48 ± 1 | 42 ± 1* | 38 ± 1* | 37 ± 1* | 35 ± 1* | 42 ± 1 |
| pH | ||||||||||||
| a | 7.39 ± 0.00 | 7.40 ± 0.00 | 7.39 ± 0.00 | 7.38 ± 0.00 | 7.37 ± 0.00 | 7.38 ± 0.01 | 7.43 ± 0.01 | 7.43 ± 0.01 | 7.44 ± 0.01 | 7.44 ± 0.01 | 7.46 ± 0.01 | 7.46 ± 0.01 |
| v | 7.38 ± 0.01 | 7.35 ± 0.01 | 7.31 ± 0.02 | 7.24 ± 0.02 | 7.19 ± 0.03 | 7.32 ± 0.01 | 7.39 ± 0.00 | 7.40 ± 0.00* | 7.44 ± 0.01* | 7.44 ± 0.01* | 7.45 ± 0.00* | 7.40 ± 0.00* |
| Glucose (mmol l−1) | ||||||||||||
| a | 6.1 ± 0.3 | 6.0 ± 0.2 | 6.0 ± 0.2 | 5.9 ± 0.2 | 5.8 ± 0.1 | 5.8 ± 0.2 | 6.1 ± 0.3 | 6.2 ± 0.3 | 6.1 ± 0.3 | 6.2 ± 0.3 | 6.2 ± 0.3 | 6.1 ± 0.3 |
| v | 5.8 ± 0.2 | 5.9 ± 0.2 | 6.0 ± 0.2 | 5.9 ± 0.2 | 5.8 ± 0.2 | 5.2 ± 0.1 | 5.6 ± 0.2 | 6.0 ± 0.2 | 6.1 ± 0.3 | 6.1 ± 0.3 | 6.2 ± 0.3 | 6.0 ± 0.3 |
| Lactate (mmol l−1) | ||||||||||||
| a | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.1 | 2.1 ± 0.2 | 3.8 ± 0.7 | 3.2 ± 0.7 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.1* | 0.9 ± 0.1* | 1.0 ± 0.1* | 0.9 ± 0.1* |
| v | 1.0 ± 0.1 | 1.5 ± 0.1 | 2.0 ± 0.1 | 3.8 ± 0.3 | 6.2 ± 0.4 | 4.9 ± 0.7 | 0.9 ± 0.1 | 0.9 ± 0.1* | 0.9 ± 0.1* | 0.9 ± 0.1* | 1.0 ± 0.1* | 1.1 ± 0.1* |
| Temperaure (°C) | ||||||||||||
| v | 36.9 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.4 ± 0.1 | 37.5 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.8 ± 0.1 | 36.6 ± 0.1* | 36.6 ± 0.1* | 36.5 ± 0.1* | 36.9 ± 0.1 |
Values are means ±s.e.m. for 9 subjects. a, arterial; v, femoral venous.
Different from exercise, P < 0.05.
Passive knee-extensor exercise
Passive exercise resulted in increases in LBF, HR, SV, leg 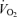 and MAP, a trend (P = 0.09) for an increase in
and MAP, a trend (P = 0.09) for an increase in 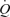 and a parallel reduction in the leg a–v O2 difference (P < 0.05; Table 2). The increase in blood flow and
and a parallel reduction in the leg a–v O2 difference (P < 0.05; Table 2). The increase in blood flow and 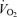 was the same at the leg and systemic levels (i.e. 0.6–0.7 l min−1 and 30–37 ml min−1, respectively). Thus, the increase in
was the same at the leg and systemic levels (i.e. 0.6–0.7 l min−1 and 30–37 ml min−1, respectively). Thus, the increase in 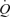 and LBF unrelated to enhanced metabolism represented only 0.3–0.4 l min−1, or ∼5% of the peak exercise leg and systemic hyperaemia (i.e. 7.2–7.9 l min−1). In support of the estimate, passive exercise only increased
and LBF unrelated to enhanced metabolism represented only 0.3–0.4 l min−1, or ∼5% of the peak exercise leg and systemic hyperaemia (i.e. 7.2–7.9 l min−1). In support of the estimate, passive exercise only increased 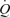 and LBF by 0.2–0.3 l min−1 when
and LBF by 0.2–0.3 l min−1 when 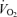 remained unchanged in Study 3.
remained unchanged in Study 3.
Table 2.
Leg and systemic haemodynamic effects of passive exercise
| Rest | Passive exercise | Peak exercise | |
|---|---|---|---|
| Leg blood flow (l min−1) | 0.61 ± 0.09 | 1.35 ± 0.17* | 7.8 ± 0.7*† |
| Leg a–v O2 difference (ml l−1) | 82 ± 9 | 61 ± 9* | 161 ± 6*† |
Leg 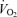 (l min−1) (l min−1) |
0.05 ± 0.01 | 0.09 ± 0.02* | 1.24 ± 0.10*† |
| Cardiac output (l min−1) | 7.7 ± 0.7 | 8.3 ± 0.6 | 15.6 ± 0.9*† |
| Heart rate (beats min−1) | 71 ± 3 | 75 ± 3* | 134 ± 5*† |
| Stroke volume (ml beat−1) | 103 ± 5 | 109 ± 5* | 113 ± 6* |
| Mean arterial pressure (mmHg) | 93 ± 4 | 95 ± 3 | 145 ± 3*† |
| Central venous pressure (mmHg) | 4 ± 1 | 5 ± 1 | 12 ± 2*† |
Pulmonary 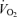 (l min−1) (l min−1) |
0.40 ± 0.01 | 0.43 ± 0.02 | 2.16 ± 0.10*† |
Values are means ±s.e.m. for 9 subjects.
Different from rest, P < 0.05.
Different from passive exercise, P < 0.05.
Arterial and venous ATP infusion
In contrast to infusion in the femoral artery, ATP infusion in the femoral vein (1 and 15 μmol min−1) did not alter LBF, 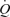 or MAP (Table 3). Likewise, LBF did not change in the non-infused leg during either femoral artery or venous ATP infusion. During recovery from exercise and arterial ATP infusion, LBF kinetics was similar.
or MAP (Table 3). Likewise, LBF did not change in the non-infused leg during either femoral artery or venous ATP infusion. During recovery from exercise and arterial ATP infusion, LBF kinetics was similar.
Table 3.
Leg and systemic haemodynamic effects of femoral artery and vein ATP infusions in resting human subjects
| Baseline | ATP infusion in femoral artery (15 μmol min−1) | Recovery 10 min | ATP infusion in femoral vein (1 μmol min−1) | ATP infusion in femoral vein (15 μmol min−1) | Recovery 10 min | |
|---|---|---|---|---|---|---|
| Leg blood flow (l min−1) | 0.7 ± 0.1 | 5.7 ± 0.5* | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1† | 0.6 ± 0.2 |
| Leg vascular conductance (ml min−1 mmHg−1) | 6.8 ± 0.7 | — | 8.8 ± 1.4* | 6.7 ± 1.1 | 7.1 ± 1.2 | 6.9 ± 0.9 |
| Cardiac output (l min−1) | 6.6 ± 0.9 | — | 6.2 ± 0.9 | 6.2 ± 1.0 | 6.8 ± 1.3 | 6.4 ± 1.0 |
| Heart rate (beats min−1) | 70 ± 5 | — | 81 ± 8* | 66 ± 5 | 75 ± 5 | 68 ± 3 |
| Stroke volume (ml beat−1) | 93 ± 8 | — | 76 ± 7* | 91 ± 2 | 88 ± 1 | 93 ± 1 |
| Mean arterial pressure (mmHg) | 98 ± 2 | — | 88 ± 8* | 97 ± 2 | 95 ± 2 | 96 ± 3 |
Values are means ±s.e.m. for 6 subjects. Leg blood flow during femoral artery ATP infusion of 1 μmol min−1 was 4.9 ± 0.2 l min−1. The missing data during arterial ATP infusion was due to the fact that arterial pressure could not be measured. Based on the results from Study 1, it estimated that 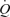 might have been ∼12 l min−1 during arterial ATP infusion, which is in large contrast to the unchanged ∼7 l min−1 during femoral venous ATP infusion. *Different from baseline, P < 0.05. †Different from arterial ATP infusion, P < 0.05.
might have been ∼12 l min−1 during arterial ATP infusion, which is in large contrast to the unchanged ∼7 l min−1 during femoral venous ATP infusion. *Different from baseline, P < 0.05. †Different from arterial ATP infusion, P < 0.05.
Cyclic external compressions
Whole thigh compressions doubled resting LBF (i.e. ΔLBF = 0.7 ± 0.1 l min−1) and vascular conductance without inducing significant alterations in HR, SV, 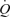 , MAP, mean femoral venous pressure or
, MAP, mean femoral venous pressure or 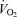 (Table 4). No differences in LBF were observed when cyclic thigh compressions were performed in the upright and supine positions (ΔLBF 0.96 ± 0.17 versus 0.75 ± 0.04 l min−1, respectively; P = 0.35). Superimposing thigh compressions upon exercise increased LBF when exercise was passive but not during voluntary exercise (Fig. 3).
(Table 4). No differences in LBF were observed when cyclic thigh compressions were performed in the upright and supine positions (ΔLBF 0.96 ± 0.17 versus 0.75 ± 0.04 l min−1, respectively; P = 0.35). Superimposing thigh compressions upon exercise increased LBF when exercise was passive but not during voluntary exercise (Fig. 3).
Table 4.
Leg and systemic haemodynamic effects of thigh compressions
| Rest | Thigh compressions | Rest | Passive exercise | Passive exercise + thigh compressions | Rest | Exercise | Exercise + thigh compressions | |
|---|---|---|---|---|---|---|---|---|
| Leg blood flow (l min−1) | 0.54 ± 0.05 | 1.23 ± 0.12* | 0.51 ± 0.03 | 0.72 ± 0.06 | 1.23 ± 0.12*† | 0.54 ± 0.05 | 1.94 ± 0.25* | 2.16 ± 0.23* |
| Leg vascular conductance (units) | 5.0 ± 0.4 | 11.1 ± 1.2* | 4.7 ± 0.3 | 6.5 ± 0.5 | 11.0 ± 1.0*† | 4.8 ± 0.5 | 16.4 ± 1.9* | 17.7 ± 1.8* |
| Leg a-v O2 difference (ml l−1) | 79 ± 11 | 72 ± 10 | 78 ± 14 | 76 ± 13 | 69 ± 13 | 75 ± 10 | 126 ± 6* | 123 ± 7* |
Leg 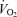 (l min−1) (l min−1) |
0.04 ± 0.01 | 0.09 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.08 ± 0.01 | 0.04 ± 0.01 | 0.24 ± 0.02* | 0.26 ± 0.04* |
| Cardiac output (l min−1) | 4.5 ± 0.3 | 4.8 ± 0.4 | 4.5 ± 0.4 | 4.6 ± 0.3 | 4.9 ± 0.2 | 4.4 ± 0.3 | 6.0 ± 0.2* | 6.5 ± 0.3* |
| Heart rate (beats min−1) | 66 ± 3 | 68 ± 3 | 66 ± 3 | 66 ± 3 | 68 ± 2 | 65 ± 3 | 79 ± 2* | 85 ± 3* |
| Stroke volume (ml beat−1) | 69 ± 4 | 69 ± 3 | 68 ± 3 | 70 ± 3 | 73 ± 4 | 68 ± 3 | 75 ± 3* | 79 ± 3* |
| Mean arterial pressure (mmHg) | 110 ± 4 | 112 ± 4 | 109 ± 3 | 111 ± 3 | 112 ± 3 | 112 ± 3 | 118 ± 5* | 123 ± 5* |
| Mean femoral vein pressure (mmHg) | 9.7 ± 1.6 | 9.9 ± 1.4 | 9.6 ± 1.5 | 10.0 ± 1.5 | 10.3 ± 1.6 | 9.9 ± 1.5 | 12.2 ± 1.9* | 13.3 ± 1.9* |
Pulmonary 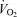 (l min−1) (l min−1) |
0.21 ± 0.02 | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.24 ± 0.01 | 0.21 ± 0.01 | 0.46 ± 0.07* | 0.49 ± 0.08* |
Values are means ±s.e.m. for 6 subjects.
Different from the corresponding resting control values, P < 0.05.
Different from passive exercise, P < 0.05. Note that the increase in leg blood flow with thigh compressions was attenuated during active exercise compared to rest (i.e. 0.2 ± 0.1 versus 0.7 ± 0.1 l min−1, respectively; P < 0.001).
Figure 3. Haemodynamic effects of exercise, ATP infusion and external compressions.
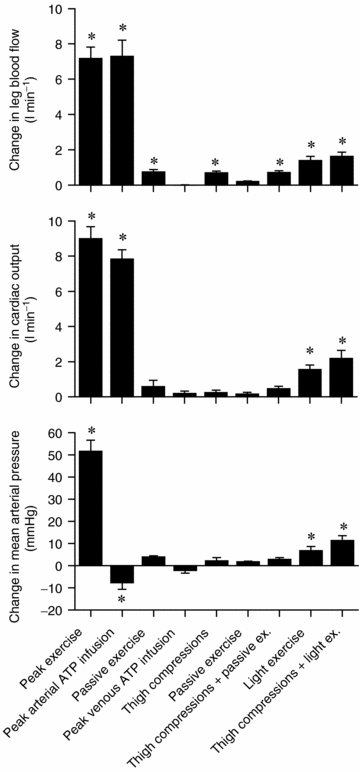
Change in blood flow and arterial pressure from baseline values during peak and light exercise, intrafemoral artery or vein ATP infusion, and thigh compressions alone and in combination with passive exercise or light exercise. Note that leg vasodilatation with arterial ATP infusion matches exercise hyperaemia, but not the increase in arterial blood pressure. ATP acts locally as intrafemoral vein ATP infusion did not alter these responses. Further, effect of mechanical-induced vasodilatation via cyclic external compressions at the same rate as exercise induces significant increases in leg and systemic blood flow, but this increase only accounts for ∼10% of the peak exercise hyperaemia. Data are mean ±s.e.m.*Significant change from corresponding baseline values, P < 0.05.
Discussion
We compared the haemodynamic responses to one-legged knee-extensor exercise, intravascular ATP infusion and external thigh compressions to gain insight into the role of the muscle pump and vasodilatory mechanisms on cardiovascular function during exercise. A major finding was that arterial ATP infusion mimicked the increases in 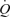 and LBF observed during incremental knee-extensor exercise without altering CVP or muscle metabolism. In contrast, infusion of ATP in the femoral vein did not change
and LBF observed during incremental knee-extensor exercise without altering CVP or muscle metabolism. In contrast, infusion of ATP in the femoral vein did not change 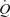 , LBF or MAP, suggesting that skeletal muscle vasodilatation drives the increase in
, LBF or MAP, suggesting that skeletal muscle vasodilatation drives the increase in 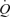 during exercise. Another salient finding was that passive knee-extensor exercise evoked a small increase in leg muscle and systemic perfusion, which was partly related to an enhanced muscle aerobic metabolism. Likewise, thigh compressions alone or in combination with passive exercise, but not voluntary exercise, doubled LBF without altering
during exercise. Another salient finding was that passive knee-extensor exercise evoked a small increase in leg muscle and systemic perfusion, which was partly related to an enhanced muscle aerobic metabolism. Likewise, thigh compressions alone or in combination with passive exercise, but not voluntary exercise, doubled LBF without altering 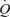 , MAP or
, MAP or 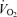 . Collectively, these findings suggest that the skeletal muscle pump is not obligatory for sustaining venous return, CVP, SV and
. Collectively, these findings suggest that the skeletal muscle pump is not obligatory for sustaining venous return, CVP, SV and 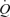 or maintaining muscle blood flow during one-legged exercise in humans. Further, the contribution of the muscle pump and mechanically induced vasodilatation to local and systemic peak exercise hyperaemia appears to be small in comparison to the effects of limb muscle vasodilatation.
or maintaining muscle blood flow during one-legged exercise in humans. Further, the contribution of the muscle pump and mechanically induced vasodilatation to local and systemic peak exercise hyperaemia appears to be small in comparison to the effects of limb muscle vasodilatation.
Cardiovascular function during exercise and pharmacologically induced vasodilatation
During exercise, there was the expected increase in 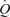 , LBF, MAP and leg and systemic O2 delivery and
, LBF, MAP and leg and systemic O2 delivery and 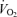 , which returned to baseline values after 10 min of recovery. The rate of increase in LBF, O2 delivery and leg
, which returned to baseline values after 10 min of recovery. The rate of increase in LBF, O2 delivery and leg 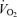 was similar to that reported during one-legged knee-extensor exercise (Andersen & Saltin, 1985; Richardson et al. 1995; Rådegran, 1997; González-Alonso et al. 2002). The increase in LBF during incremental exercise matched the increase in
was similar to that reported during one-legged knee-extensor exercise (Andersen & Saltin, 1985; Richardson et al. 1995; Rådegran, 1997; González-Alonso et al. 2002). The increase in LBF during incremental exercise matched the increase in 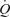 , indicating that the augmented systemic variables reflected the enhanced metabolic demand of the exercising knee-extensor muscles (Savard et al. 1988; Magnusson et al. 1994; González-Alonso et al. 2006). In other words, global blood flow to the upper body and the contralateral leg remained unchanged with only a tendency to increase at peak exercise when regional
, indicating that the augmented systemic variables reflected the enhanced metabolic demand of the exercising knee-extensor muscles (Savard et al. 1988; Magnusson et al. 1994; González-Alonso et al. 2006). In other words, global blood flow to the upper body and the contralateral leg remained unchanged with only a tendency to increase at peak exercise when regional 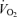 increased. The close matching between O2 supply and metabolic demand was therefore mediated largely by local quadriceps muscle vascular mechanisms, which are thought to involve mechanical, neurological and metabolic signals (Guyton et al. 1962; Laughlin et al. 1996; Rowell, 2004; Saltin, 2007).
increased. The close matching between O2 supply and metabolic demand was therefore mediated largely by local quadriceps muscle vascular mechanisms, which are thought to involve mechanical, neurological and metabolic signals (Guyton et al. 1962; Laughlin et al. 1996; Rowell, 2004; Saltin, 2007).
An important observation was that step-wise femoral artery infusion of ATP evoked the same increase in 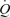 and LBF as incremental exercise, albeit the contribution of HR and SV to
and LBF as incremental exercise, albeit the contribution of HR and SV to 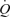 differed markedly. During incremental exercise, HR increased in a curvilinear fashion from 64 to 134 beats min−1, driving all the increase in
differed markedly. During incremental exercise, HR increased in a curvilinear fashion from 64 to 134 beats min−1, driving all the increase in 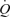 above 30% of peak power as SV plateaued or tended to decline (Åstrand et al. 1964; Poliner et al. 1980; Keul et al. 1981; Higginbotham et al. 1986; Mortensen et al. 2005). In contrast, the same increase in
above 30% of peak power as SV plateaued or tended to decline (Åstrand et al. 1964; Poliner et al. 1980; Keul et al. 1981; Higginbotham et al. 1986; Mortensen et al. 2005). In contrast, the same increase in 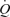 with graded arterial ATP infusion was associated with both progressive elevations in HR (70–95 beats min−1) and gradual increases in SV (103–155 ml beat−1). Consequently, SV was higher during arterial ATP infusion at the two highest levels of
with graded arterial ATP infusion was associated with both progressive elevations in HR (70–95 beats min−1) and gradual increases in SV (103–155 ml beat−1). Consequently, SV was higher during arterial ATP infusion at the two highest levels of 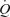 , suggesting that tachycardia blunted the increase in SV during exercise. This is in agreement with findings on heart transplant patients demonstrating that the SV response to exercise is augmented because their HR is retarded, but the rise in
, suggesting that tachycardia blunted the increase in SV during exercise. This is in agreement with findings on heart transplant patients demonstrating that the SV response to exercise is augmented because their HR is retarded, but the rise in 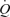 with respect to metabolic demand is normal (Stinson et al. 1972; Clark et al. 1973; Verani et al. 1994). Another striking observation was that systemic and leg vascular conductance was much greater at the two highest levels of hyperaemia during ATP infusion than during exercise. The lack of vasoconstriction in the leg and other bodily tissues during ATP infusion appears to account for this response. These observations, on one hand, support the idea that peripheral, rather than central factors, control the circulatory responses to exercise (Guyton et al. 1962; Laughlin et al. 1996; Rowell, 2004; Rowland, 2005) and, on the other hand, cast doubts about the necessity of the muscle pump to increase and maintain SV and
with respect to metabolic demand is normal (Stinson et al. 1972; Clark et al. 1973; Verani et al. 1994). Another striking observation was that systemic and leg vascular conductance was much greater at the two highest levels of hyperaemia during ATP infusion than during exercise. The lack of vasoconstriction in the leg and other bodily tissues during ATP infusion appears to account for this response. These observations, on one hand, support the idea that peripheral, rather than central factors, control the circulatory responses to exercise (Guyton et al. 1962; Laughlin et al. 1996; Rowell, 2004; Rowland, 2005) and, on the other hand, cast doubts about the necessity of the muscle pump to increase and maintain SV and 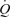 during exercise.
during exercise.
Muscle pump and cardiovascular control
Conventionally, the muscle pump is thought to play an important role in maintaining CVP, end-diastolic volume and SV when 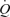 is increased during exercise (Notarius & Magder, 1996). Guyton et al. (1962) and Rowell (1992, 1993, 2004) argue that translocation of blood volume from the vasculature to the heart increases
is increased during exercise (Notarius & Magder, 1996). Guyton et al. (1962) and Rowell (1992, 1993, 2004) argue that translocation of blood volume from the vasculature to the heart increases 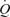 because the heart itself cannot raise its output to the level produced during exercise. In line with this notion, Sheriff et al. (1993) showed that the increase in HR with ventricular pacing was accompanied by a fall in CVP and SV, such that
because the heart itself cannot raise its output to the level produced during exercise. In line with this notion, Sheriff et al. (1993) showed that the increase in HR with ventricular pacing was accompanied by a fall in CVP and SV, such that 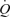 increased only slightly. They also showed that similar attempts to raise
increased only slightly. They also showed that similar attempts to raise  above its normal level in voluntarily exercising dogs were counteracted by a fall in CVP and SV. According to Rowell (1992, 2004), these findings of Sheriff et al. support the need for the muscle pump to counteract the flow-dependent redistribution of blood volume from the central to peripheral vessels and thereby prevent the negative effects of increased
above its normal level in voluntarily exercising dogs were counteracted by a fall in CVP and SV. According to Rowell (1992, 2004), these findings of Sheriff et al. support the need for the muscle pump to counteract the flow-dependent redistribution of blood volume from the central to peripheral vessels and thereby prevent the negative effects of increased  on end-diastolic volume of the heart and SV. The applicability of these findings in instrumented animals to exercising humans remains debated (Laughlin & Joyner, 2003; Magder, 2005; Brengelmann, 2005; Clifford et al. 2005; Rothe, 2005; Sheriff, 2005), particularly in the light of the observations that CVP does not need to decline during incremental upright whole-body exercise in humans (Mortensen et al. 2005), nor during incremental knee-extensor exercise, passive knee-extensor exercise or ATP infusion (Fig. 2). Arguing against a crucial role of the muscle pump in the cardiovascular responses to exercise, patients with a congenital absence of valves in the deep veins of the legs also show an increase in SV and
on end-diastolic volume of the heart and SV. The applicability of these findings in instrumented animals to exercising humans remains debated (Laughlin & Joyner, 2003; Magder, 2005; Brengelmann, 2005; Clifford et al. 2005; Rothe, 2005; Sheriff, 2005), particularly in the light of the observations that CVP does not need to decline during incremental upright whole-body exercise in humans (Mortensen et al. 2005), nor during incremental knee-extensor exercise, passive knee-extensor exercise or ATP infusion (Fig. 2). Arguing against a crucial role of the muscle pump in the cardiovascular responses to exercise, patients with a congenital absence of valves in the deep veins of the legs also show an increase in SV and 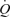 during exercise whilst CVP is maintained or slightly reduced (Bevegård & Lodin, 1962). Therefore, the current data with arterial ATP infusion challenge the muscle pump hypothesis by demonstrating that SV can increase even above exercise levels and
during exercise whilst CVP is maintained or slightly reduced (Bevegård & Lodin, 1962). Therefore, the current data with arterial ATP infusion challenge the muscle pump hypothesis by demonstrating that SV can increase even above exercise levels and 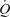 can increase to 15 l min−1 to account for the
can increase to 15 l min−1 to account for the 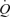 of humans with a
of humans with a 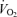 of ∼2 l min−1 without activation of the skeletal muscle pump.
of ∼2 l min−1 without activation of the skeletal muscle pump.
Muscle vasodilatory mechanisms and cardiovascular control
Another significant observation was that passive knee-extensor exercise and cyclic thigh compressions increased LBF but the magnitude of the response was small compared to the values at peak exercise. For instance, the increase in LBF and 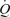 during passive exercise was 0.6–0.7 l min−1 (representing a 120% increase in LBF) and was associated with a 73% increase in muscle
during passive exercise was 0.6–0.7 l min−1 (representing a 120% increase in LBF) and was associated with a 73% increase in muscle 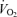 . This value agrees closely with the ∼0.5 l min−1 LBF increase found during passive supine and upright knee-extensor exercise (Wray et al. 2005), but is higher than the 0.3 l min−1 increase we found when leg
. This value agrees closely with the ∼0.5 l min−1 LBF increase found during passive supine and upright knee-extensor exercise (Wray et al. 2005), but is higher than the 0.3 l min−1 increase we found when leg 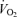 did not increase during passive exercise in Study 3. Thus, these findings during passive exercise point towards a small independent contribution of mechanical factors to exercise hyperaemia. Passive leg motions alone do not adequately simulate activation of the muscle pump, which involves squeezing the blood out of the veins and venules of the dependent leg. In a subsequent study, we therefore applied external cyclic compressions to the whole thigh via inflation of a cuff to 200 mmHg at a rate of 60 Hz to increase intramuscular pressure to peak leg-kicking exercise levels. This was done independently and in combination with passive and voluntary knee-extensor exercise.
did not increase during passive exercise in Study 3. Thus, these findings during passive exercise point towards a small independent contribution of mechanical factors to exercise hyperaemia. Passive leg motions alone do not adequately simulate activation of the muscle pump, which involves squeezing the blood out of the veins and venules of the dependent leg. In a subsequent study, we therefore applied external cyclic compressions to the whole thigh via inflation of a cuff to 200 mmHg at a rate of 60 Hz to increase intramuscular pressure to peak leg-kicking exercise levels. This was done independently and in combination with passive and voluntary knee-extensor exercise.
In keeping with the passive exercise results, we found that whole thigh cyclic compressions alone and in combination with passive exercise, but not with voluntary exercise, doubled LBF (ΔLBF 0.5–0.7 l min−1) without significantly altering other cardiovascular variables. A possible explanation for the lack of effect during voluntary exercise might be that the additional compression did not have any influence on venous return since compressions were applied when the veins were already empty (Tschakovsky et al. 2004). These findings, however, are in close agreement with a study in the human forearm demonstrating a 2- to 3-fold increase in forearm blood flow and vascular conductance immediately after single, sustained and repeated whole forearm compressions (Kirby et al. 2007). The present observations in the thigh support a contribution of mechanical influences to exercise hyperaemia in the human leg (Clifford et al. 2006; Dufour et al. 2006; Kirby et al. 2007). Although the relative responses in the upper and lower limbs appear to be similar, the absolute increase in blood flow is much greater in the leg than in the forearm. This makes it possible to assess the central haemodynamic effects of external compressions in this study. Interestingly, the increases in LBF and 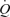 in all experimental conditions were highly correlated to increases in leg
in all experimental conditions were highly correlated to increases in leg 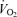 (slopes, 6.1 and 7.1 l min−1
(slopes, 6.1 and 7.1 l min−1 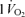 −1, respectively; Fig. 4), suggesting that the major contributor to local and systemic blood flow during one-legged kicking exercise is oxygen-related vasodilatation. We estimated that the increase in LBF and
−1, respectively; Fig. 4), suggesting that the major contributor to local and systemic blood flow during one-legged kicking exercise is oxygen-related vasodilatation. We estimated that the increase in LBF and 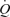 during passive exercise and thigh compressions, unrelated to the rate of muscle metabolism, accounts for ∼5% of peak knee-extensor exercise or peak ATP-induced hyperaemia (Figs 3 and 4). This is in congruence with recent findings showing that the increase in forearm blood flow and vascular conductance during repeated forearm compressions (200 mmHg) only accounted for ∼20% of contraction-induced hyperaemia (Kirby et al. 2007). The failure of cyclic thigh compressions to elicit a larger increase in LBF in the upright position where the veins are loaded compared to the supine position where they are unloaded is another important piece of evidence refuting a significant contribution of the skeletal muscle pump. Collectively, these findings during cyclic thigh compressions and passive knee-extensor exercise suggest that the muscle pump and mechanically induced vasodilatation account for a small fraction of the leg and systemic exercise hyperaemia.
during passive exercise and thigh compressions, unrelated to the rate of muscle metabolism, accounts for ∼5% of peak knee-extensor exercise or peak ATP-induced hyperaemia (Figs 3 and 4). This is in congruence with recent findings showing that the increase in forearm blood flow and vascular conductance during repeated forearm compressions (200 mmHg) only accounted for ∼20% of contraction-induced hyperaemia (Kirby et al. 2007). The failure of cyclic thigh compressions to elicit a larger increase in LBF in the upright position where the veins are loaded compared to the supine position where they are unloaded is another important piece of evidence refuting a significant contribution of the skeletal muscle pump. Collectively, these findings during cyclic thigh compressions and passive knee-extensor exercise suggest that the muscle pump and mechanically induced vasodilatation account for a small fraction of the leg and systemic exercise hyperaemia.
Figure 4. Leg blood flow and blood pressure as a function of leg aerobic metabolism.
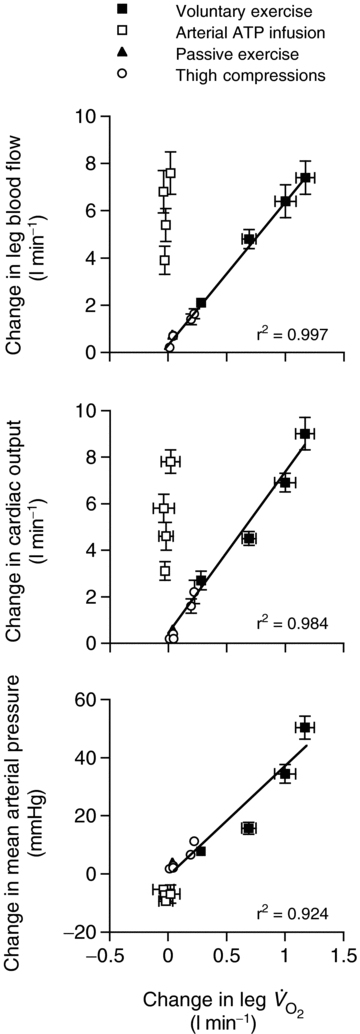
Changes in blood flow and arterial pressure from baseline values during incremental knee-extensor exercise, step-wise intrafemoral artery ATP infusion, passive exercise and thigh compressions alone and in combination with passive exercise or light exercise. Noteworthy is the significant correlations between the increases in leg blood flow, cardiac output and mean arterial pressure and the increases in leg 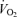 during the exercise and thigh compressions protocols, but the dissociation in these responses during arterial ATP infusion.
during the exercise and thigh compressions protocols, but the dissociation in these responses during arterial ATP infusion.
Leg muscle hyperaemia during both exercise and arterial ATP infusion is brought about by local vasodilatation overriding concomitant augmented sympathetic vasoconstrictor outflow (Von Euler, 1974; Joyner & Halliwill, 2000; Rosenmeier et al. 2004; Ichinose et al. 2006). During exercise, however, sympathetic activation is associated with robust increases in HR and MAP (the ‘exercise pressor reflex’; Fig. 1) whereas during arterial ATP and adenosine infusions, MAP remains unchanged, or declines slightly, and the HR response is blunted in association with a higher SV as discussed above (Rådegran & Hellsten, 2000; Rådegran & Calbet, 2001; Rosenmeier et al. 2004) (Fig. 4). Intravascular ATP infusion does not enhance muscle metabolism or plasma ATP, nor does it activate muscle contraction. It is therefore doubtful that metabolic by-products and ATP accumulate in the interstitium and chemo- and mechanoreceptors are activated with ATP infusion (González-Alonso et al. 2002; Rosenmeier et al. 2004; SP Mortensen, J González-Alonso, L Brune, B Saltin & H Hellsten, unpublished observation). The distinct blood pressure response during exercise might be associated with activation of mechanosensitive afferent type III fibres and chemosensitive afferent type IV fibres (Mitchell et al. 1989; Kindig et al. 2007). The similar blood flows but very different blood pressure responses during arterial ATP infusion and exercise suggest distinct regulatory loci and signalling pathways in human skeletal muscle for blood flow and blood pressure regulation.
The present results raise the question of how is preload to the heart maintained in the ATP condition? As discussed above, neither arterial nor venous ATP infusion is likely to stimulate the metaboreflex and, given the lack of change in MAP and CVP with increasing infusion rate of ATP, it is also unlikely that arterial or cardiopulmonary baroreceptors are stimulated to a significant extent. The possibility that vasoconstriction in other vascular beds maintains preload to the heart by ATP infusion is also at odds with the observations that: (i) the control leg blood flow and vascular conductance are unchanged with arterial ATP infusion (González-Alonso et al. 2002; Fig. 3), (ii) non-exercising and non-infused bodily tissues blood flow (difference between 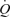 and LBF) is the same with graded ATP infusion and exercise (Fig. 2), and (iii) non-exercising and non-infused tissue vascular conductance remained unchanged with increasing rates of ATP infusion. Apart from the robust vasodilator response (27-fold increase in leg blood flow and vascular conductance) and significant increase in HR, graded arterial ATP infusion results in a 2.4-fold increase in muscle sympathetic nerve activity and circulating noradrenaline (Rosenmeier et al. 2004). Here, we found that venous ATP infusion did not alter leg or systemic haemodynamics and since HR did not change, it is likely that sympathetic activity was also unaltered (Table 3). Hence, it is unlikely that intravascular ATP infusion is increasing
and LBF) is the same with graded ATP infusion and exercise (Fig. 2), and (iii) non-exercising and non-infused tissue vascular conductance remained unchanged with increasing rates of ATP infusion. Apart from the robust vasodilator response (27-fold increase in leg blood flow and vascular conductance) and significant increase in HR, graded arterial ATP infusion results in a 2.4-fold increase in muscle sympathetic nerve activity and circulating noradrenaline (Rosenmeier et al. 2004). Here, we found that venous ATP infusion did not alter leg or systemic haemodynamics and since HR did not change, it is likely that sympathetic activity was also unaltered (Table 3). Hence, it is unlikely that intravascular ATP infusion is increasing 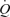 through a direct effect of circulating ATP on the heart. This implies that increases in venous return, SV and
through a direct effect of circulating ATP on the heart. This implies that increases in venous return, SV and 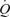 with ATP infusion might involve mechanisms linking the functioning of skeletal and cardiac muscle, independently of the established contraction-activated mechanical, neurological and metabolic signalling pathways.
with ATP infusion might involve mechanisms linking the functioning of skeletal and cardiac muscle, independently of the established contraction-activated mechanical, neurological and metabolic signalling pathways.
In conclusion, this study provides evidence in humans supporting the idea that the muscle pump is not necessary for sustaining venous return, CVP and 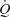 and maintaining muscle blood flow during one-legged exercise. Further, the contribution of the muscle pump and mechanically induced vasodilatation to local and systemic peak exercise hyperaemia appears to be small in comparison to the effects of limb muscle vasodilatation.
and maintaining muscle blood flow during one-legged exercise. Further, the contribution of the muscle pump and mechanically induced vasodilatation to local and systemic peak exercise hyperaemia appears to be small in comparison to the effects of limb muscle vasodilatation.
Acknowledgments
We give special thanks to the volunteer subjects. We also thank Troels Munch, Jacob Mørkeberg, Peter Nissen, James Pearson, Eric Stöhr, David Low and Dr Lotlikar for their assistance. Special thanks are given to Roger Paton from the Brunel Institute of Bioengineering for his help with the cuff inflation–deflation unit. The studies were supported by the Novo Nordisk Foundation, The Copenhagen Hospital System and Brunel University.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åstrand PO, Cuddy TE, Saltin B, Stenberg J. Cardiac output during submaximal and maximal work. J Appl Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- Bevegård S, Lodin A. Postural circulatory changes at rest and during exercise in five patients with congenital absence of valves in the deep veins of the legs. Acta Med Scand. 1962;172:21–29. doi: 10.1111/j.0954-6820.1962.tb07124.x. [DOI] [PubMed] [Google Scholar]
- Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol. 2005;90:437–446. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- Brengelmann GL. Counterpoint: the classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is not correct. J Appl Physiol. 2005;101:1525–1526. doi: 10.1152/japplphysiol.00698a.2006. [DOI] [PubMed] [Google Scholar]
- Clark DA, Schroeder JS, Griepp RB, Stinson EB, Dong E, Shumway NE, Harrison DC. Cardiac transplantation in man. Review of first three years’ experience. Am J Med. 1973;54:563–576. doi: 10.1016/0002-9343(73)90114-9. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Hamann JJ, Valic Z, Buckwalter JB. Counterpoint: The muscle pump is not an important determinant of muscle blood flow during exercise. J Appl Physiol. 2005;99:372–374. [PubMed] [Google Scholar]
- Clifford PS, Klues HA, Hammann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol. 2006;572:561–567. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson JL, Gladden LB. Effect of rhythmic titanic skeletal muscle contractions on peak muscle perfusion. J Appl Physiol. 2003;94:11–19. doi: 10.1152/japplphysiol.00339.2002. [DOI] [PubMed] [Google Scholar]
- Dufour SP, Doutreleau S, Lonsdorfer-Wolf E, Lampert E, Hirth C, Piquard F, Lonsdorfer J, Geny B, Mettauer B, Richard R. Deciphering the metabolic and mechanical contributions to the exercise-induced circulatory response: insights from eccentric cycling. Am J Physiol Regul Integr Comp Physiol. 2006;292:R1641–R1648. doi: 10.1152/ajpregu.00567.2006. [DOI] [PubMed] [Google Scholar]
- Folkow J, Haglund U, Jodal M, Lundgren O. Blood flow in the calf muscle of man during heavy rhythmic exercise. Acta Physiol Scand. 1971;80:61–72. doi: 10.1111/j.1748-1716.1971.tb04887.x. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol. 2000;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton AC, Douglas BH, Langston JB, Richardson TQ. Instantaneous increase in mean circulatory pressure and cardiac output at onset of muscular activity. Circ Res. 1962;11:431–441. doi: 10.1161/01.res.11.3.431. [DOI] [PubMed] [Google Scholar]
- Hamann JJ, Valic Z, Buckwalter JB, Clifford PS. Muscle pump does not enhance blood flow in exercising skeletal muscle. J Appl Physiol. 2003;94:6–10. doi: 10.1152/japplphysiol.00337.2002. [DOI] [PubMed] [Google Scholar]
- Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Kondo N, Nishiyasu T. Time-dependent modulation of arterial baroreflex control of muscle sympathetic nerve activity during isometric exercise in humans. Am J Physiol Heart Circ Physiol. 2006;290:H1419–H1426. doi: 10.1152/ajpheart.00847.2005. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Halliwill JR. Sympathetic vasodilatation in human limbs. J Physiol. 2000;526:471–480. [PubMed] [Google Scholar]
- Keul J, Dickhuth HH, Simon G, Lehmann M. Effect of static and dynamic exercise on heart volume, contractility, and left ventricular dimensions. Circ Res. 1981;48:I162–I1170. [PubMed] [Google Scholar]
- Kindig AE, Hayes SG, Kaufman MP. Purinergic 2 receptor blockade prevents the responses of group IV afferents to post-contraction circulatory occlusion. J Physiol. 2007;578:301–308. doi: 10.1113/jphysiol.2006.119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno F. Mechanical influences on skeletal muscle tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol. 2007;583:861–874. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in excercise hyperemia. Am J Physiol. 1987;253:H993–1004. doi: 10.1152/ajpheart.1987.253.5.H993. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Joyner M. Closer to the edge? Contractions, pressures, waterfalls and blood flow to contracting skeletal muscle. J Appl Physiol. 2003;94:3–15. doi: 10.1152/japplphysiol.00829.2002. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD, USA: American Physiological Society; 1996. pp. 705–769. chap. 16. [Google Scholar]
- Laughlin MH, Schrage WG. Effects of muscle contraction on skeletal muscle blood flow: when there is a muscle pump? Med Sci Sport Exerc. 1999;31:1027–1035. doi: 10.1097/00005768-199907000-00016. [DOI] [PubMed] [Google Scholar]
- Magder S. The classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance governs venous return is/is not correct. J Appl Physiol. 2005;101:1533. doi: 10.1152/japplphysiol.00903.2006. [DOI] [PubMed] [Google Scholar]
- Magnusson G, Kaijser L, Isberg B, Saltin B. Cardiovascular responses during one- and two-legged exercise in middle-aged men. Acta Physiol Scand. 1994;150:353–362. doi: 10.1111/j.1748-1716.1994.tb09699.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR, Jr, Rogers HB, Secher NH. Epidural anaesthesia and cardiovascular responses to static exercise in man. J Physiol. 1989;417:13–24. doi: 10.1113/jphysiol.1989.sp017787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, González-Alonso J. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol. 2005;566:273–285. doi: 10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarius CF, Magder S. Central venous pressure during exercise: role of muscle pump. Can J Physiol Pharmacol. 1996;74:647–651. [PubMed] [Google Scholar]
- Poliner LR, Dehmer GJ, Lewis SE, Parkey RW, Blomqvist CG, Willerson JT. Left ventricular performance in normal subjects: a comparison of the responses to exercise in the upright and supine positions. Circulation. 1980;62:528–534. doi: 10.1161/01.cir.62.3.528. [DOI] [PubMed] [Google Scholar]
- Pollack AA, Wood EH. Venous pressure in the saphenous vein at the ankle in man during exercise and changes in posture. J Appl Physiol. 1949;1:649–662. doi: 10.1152/jappl.1949.1.9.649. [DOI] [PubMed] [Google Scholar]
- Rådegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee-extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilation. Acta Physiol Scand. 2001;171:177–185. doi: 10.1046/j.1365-201x.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Hellsten Y. Adenosine and nitric oxide in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand. 2000;168:575–591. doi: 10.1046/j.1365-201x.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise: evidence of limited O2 transport. J Clin Invest. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe CF. The muscle pump indeed raises muscle blood flow during locomotion. J Appl Physiol. 2005;99:773. doi: 10.1152/japplphysiol.00602.2005. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Reflex control of the circulation during exercise. Int J Sports Med. 1992;13(Suppl. 1):S25–S27. doi: 10.1055/s-2007-1024583. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. Central circulatory adjustments to dynamic exercise. [Google Scholar]
- Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol. 2004;97:384–392. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- Rowell LB, O’Leary DS, Kellong DL. Integration of cardiovascular control systems in dynamic exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD, USA: American Physiological Society; 1996. pp. 771–781. [Google Scholar]
- Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol. 1986;251:H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- Rowland TW. Circulatory responses to exercise: are we misreading Fick? Chest. 2005;127:1023–1030. doi: 10.1378/chest.127.3.1023. [DOI] [PubMed] [Google Scholar]
- Saltin B. Exercise hyperaemia: magnitude and aspects on regulation in humans. J Physiol. 2007;586:123–130. doi: 10.1113/jphysiol.2007.136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard GK, Nielsen B, Laszczynska J, Larsen BE, Saltin B. Muscle blood flow is not reduced in humans during moderate exercise and heat stress. J Appl Physiol. 1988;64:649–657. doi: 10.1152/jappl.1988.64.2.649. [DOI] [PubMed] [Google Scholar]
- Shepherd JT. Circulation to skeletal muscle. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol. III, Peripheral Circulation and Organ Blood Flow. Bethesda, MD, USA: American Physiological Society; 1983. pp. 319–370. [Google Scholar]
- Sheriff D. Point: The muscle pump raises muscle blood flow during locomotion. J Appl Physiol. 2005;99:371–372. doi: 10.1152/japplphysiol.00381.2005. discussion 374–375. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol Heart Circ Physiol. 1993;265:H1227–H1234. doi: 10.1152/ajpheart.1993.265.4.H1227. [DOI] [PubMed] [Google Scholar]
- Shiotani I, Sato H, Yokoyama H, Ohnishi Y, Hishida E, Kinjo K, Nakatani D, Kuzuya T, Hori M. Muscle pump-dependent self-perfusion mechanism in legs in normal subjects and patients with heart failure. J Appl Physiol. 2002;92:1647–1654. doi: 10.1152/japplphysiol.01096.2000. [DOI] [PubMed] [Google Scholar]
- Stinson EB, Griepp RB, Schroeder JS, Dong E, Jr, Shumway NE. Hemodynamic observations one and two years after cardiac transplantation in man. Circulation. 1972;45:1183–1194. doi: 10.1161/01.cir.45.6.1183. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilatation. J Appl Physiol. 2004;96:639–644. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- Verani MS, Nishimura S, Mahmarian JJ, Hays JT, Young JB. Cardiac function after orthotopic heart transplantation: response to postural changes, exercise, and b-adrenergic blockade. J Heart Lung Transplant. 1994;13:181–193. [PubMed] [Google Scholar]
- Von Euler US. Sympatho-adrenal activity in physical exercise. Med Sci Sports. 1974;6:165–173. [PubMed] [Google Scholar]
- Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol. 2005;565:1053–1060. doi: 10.1113/jphysiol.2005.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]


