Abstract
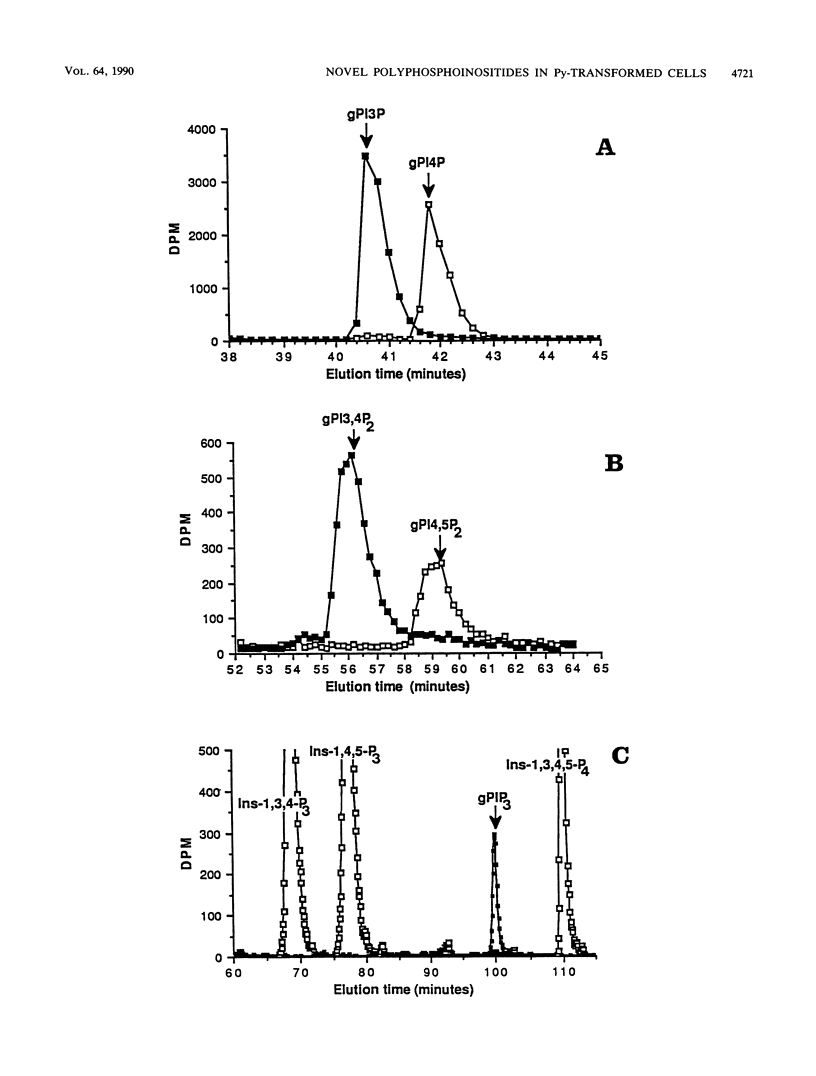
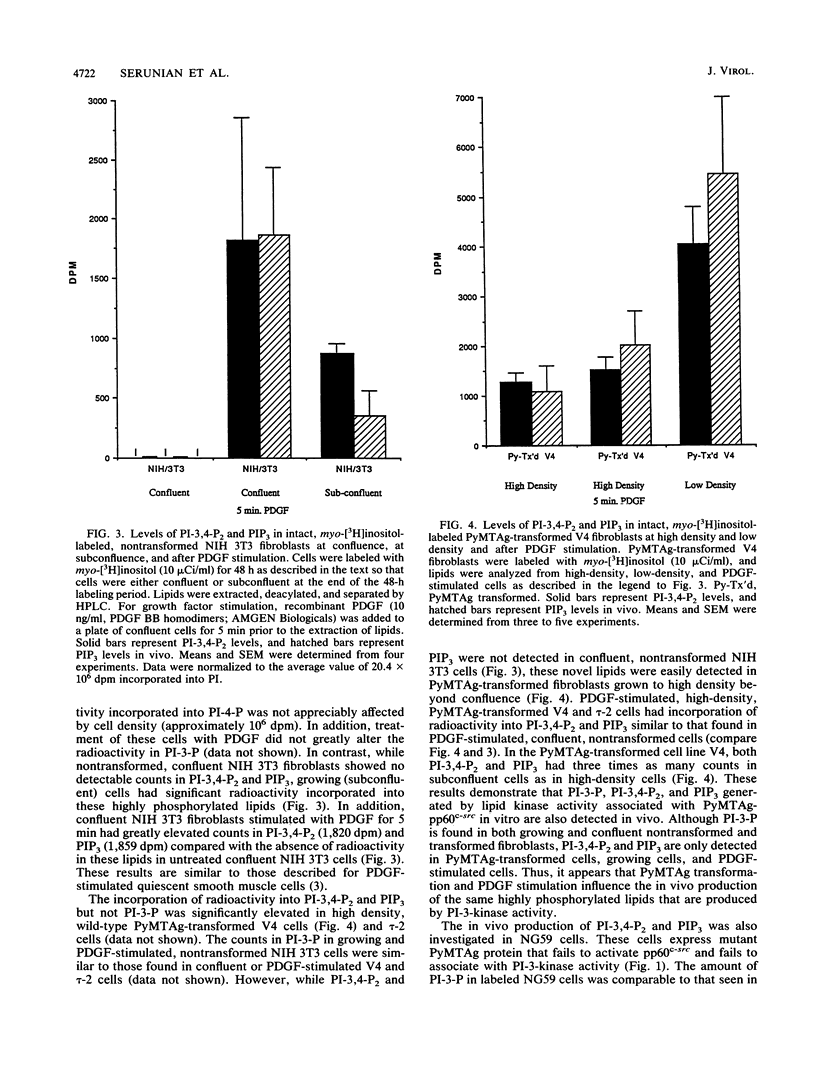
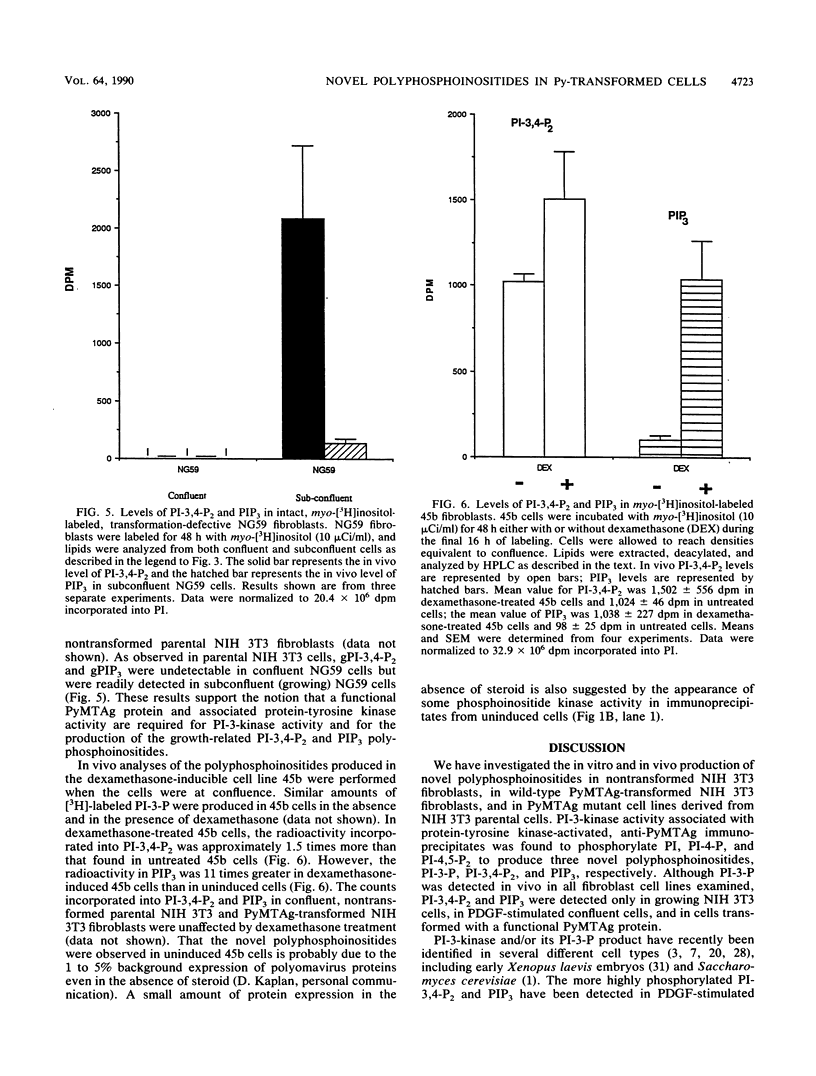
Phosphatidylinositol 3-kinase associates with the polyomavirus middle T antigen (PyMTAg)-pp60c-src complex in polyomavirus-transformed cells. Here we show that anti-PyMTAg immunoprecipitates from PyMTAg-transformed NIH 3T3 cells have lipid kinase activities that phosphorylate phosphatidylinositol, phosphatidylinositol-4-bisphosphate, and phosphatidylinositol-4,5-bisphosphate at the D-3 position of the inositol ring to produce three new polyphosphoinositides: phosphatidylinositol-3-phosphate (PI-3-P), phosphatidylinositol-3,4-bisphosphate (PI-3,4-P2), and phosphatidylinositol trisphosphate (PIP3), respectively. PI-3-P was detected in intact parental and PyMTAg-transformed NIH 3T3 fibroblasts at both low and high cell densities. However, parental NIH 3T3 fibroblasts produced no detectable PI-3,4-P2 or PIP3 at high density. In contrast, growing, subconfluent cells and wild-type PyMTAg-transformed cells at high density had greatly enhanced incorporation of [3H]-inositol into these highly phosphorylated lipids. Cells transfected with a transformation-defective mutant of PyMTAg had undetectable levels of PI-3,4-P2 and PIP3 at high density. Thus, the synthesis of novel polyphosphoinositides by lipid kinase activity associated with PyMTAg correlates with cell growth and transformation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger K. R., Carpenter C. L., Cantley L. C., Varticovski L. Phosphatidylinositol 3-kinase and its novel product, phosphatidylinositol 3-phosphate, are present in Saccharomyces cerevisiae. J Biol Chem. 1989 Dec 5;264(34):20181–20184. [PubMed] [Google Scholar]
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Nairn A. C., Kuo J. F. Protein kinases 1988: a current perspective. FASEB J. 1988 Nov;2(14):2957–2969. doi: 10.1096/fasebj.2.14.2972578. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Escobedo J. A., Williams L. T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science. 1989 Mar 3;243(4895):1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Heber A. An 81 kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell. 1987 Sep 25;50(7):1031–1037. doi: 10.1016/0092-8674(87)90169-3. [DOI] [PubMed] [Google Scholar]
- Endemann G., Yonezawa K., Roth R. A. Phosphatidylinositol kinase or an associated protein is a substrate for the insulin receptor tyrosine kinase. J Biol Chem. 1990 Jan 5;265(1):396–400. [PubMed] [Google Scholar]
- Fukui Y., Hanafusa H. Phosphatidylinositol kinase activity associates with viral p60src protein. Mol Cell Biol. 1989 Apr;9(4):1651–1658. doi: 10.1128/mcb.9.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Kornbluth S., Jong S. M., Wang L. H., Hanafusa H. Phosphatidylinositol kinase type I activity associates with various oncogene products. Oncogene Res. 1989;4(4):283–292. [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Raptis L., Garcea R. L., Pallas D., Roberts T. M., Cantley L. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3624–3628. doi: 10.1073/pnas.83.11.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lips D. L., Majerus P. W., Gorga F. R., Young A. T., Benjamin T. L. Phosphatidylinositol 3-phosphate is present in normal and transformed fibroblasts and is resistant to hydrolysis by bovine brain phospholipase C II. J Biol Chem. 1989 May 25;264(15):8759–8763. [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Raptis L., Lamfrom H., Benjamin T. L. Regulation of cellular phenotype and expression of polyomavirus middle T antigen in rat fibroblasts. Mol Cell Biol. 1985 Sep;5(9):2476–2486. doi: 10.1128/mcb.5.9.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Kapeller R., White M. F., Cantley L. C. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serunian L. A., Haber M. T., Fukui T., Kim J. W., Rhee S. G., Lowenstein J. M., Cantley L. C. Polyphosphoinositides produced by phosphatidylinositol 3-kinase are poor substrates for phospholipases C from rat liver and bovine brain. J Biol Chem. 1989 Oct 25;264(30):17809–17815. [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Downes C. P. Metabolic and structural evidence for the existence of a third species of polyphosphoinositide in cells: D-phosphatidyl-myo-inositol 3-phosphate. Biochem J. 1989 Apr 1;259(1):267–276. doi: 10.1042/bj2590267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Whitman M., Cantley L. C., Erikson R. L. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmage D. A., Freund R., Young A. T., Dahl J., Dawe C. J., Benjamin T. L. Phosphorylation of middle T by pp60c-src: a switch for binding of phosphatidylinositol 3-kinase and optimal tumorigenesis. Cell. 1989 Oct 6;59(1):55–65. doi: 10.1016/0092-8674(89)90869-6. [DOI] [PubMed] [Google Scholar]
- Traynor-Kaplan A. E., Harris A. L., Thompson B. L., Taylor P., Sklar L. A. An inositol tetrakisphosphate-containing phospholipid in activated neutrophils. Nature. 1988 Jul 28;334(6180):353–356. doi: 10.1038/334353a0. [DOI] [PubMed] [Google Scholar]
- Varticovski L., Druker B., Morrison D., Cantley L., Roberts T. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature. 1989 Dec 7;342(6250):699–702. doi: 10.1038/342699a0. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Daniel T. O., Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science. 1988 Aug 19;241(4868):968–970. doi: 10.1126/science.2457254. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Olashaw N. E., Nishibe S., Rhee S. G., Pledger W. J., Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989 Jul;9(7):2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Cantley L. Phosphoinositide metabolism and the control of cell proliferation. Biochim Biophys Acta. 1989 Feb;948(3):327–344. doi: 10.1016/0304-419x(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988 Apr 14;332(6165):644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D., Roberts T., Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987 Oct 1;247(1):165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Melton D. A. Induction of mesoderm by a viral oncogene in early Xenopus embryos. Science. 1989 May 19;244(4906):803–806. doi: 10.1126/science.2658054. [DOI] [PubMed] [Google Scholar]




