Abstract
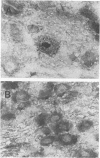
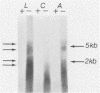
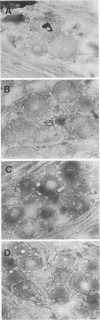
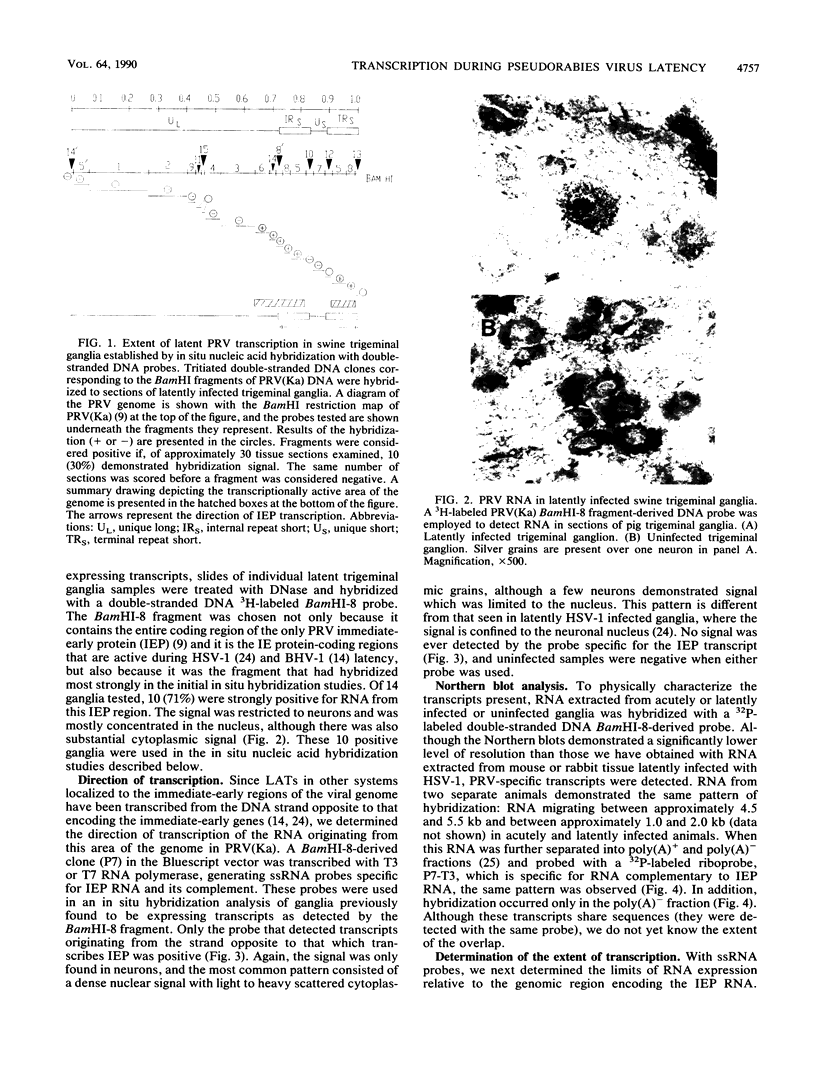
Pseudorabies virus (PRV) is a porcine herpesvirus that establishes latent infections in trigeminal ganglia. To determine whether PRV expresses any transcripts that could play a role in latency, the trigeminal ganglia of 14 pigs previously inoculated through the nose and latently infected with PRV(Ka) were assayed by in situ nucleic acid hybridization for the presence of PRV-specific RNA. Hybridizations employing probes encompassing the entire viral genome revealed that an area extending from 0.64 to 0.82 map units was transcriptionally active. The DNA probe that most consistently detected transcripts was BamHI-8, a fragment which contains the gene for the immediate-early protein. With this probe, ganglia from 10 (71%) of 14 pigs scored positive for PRV RNA, although only 1 (8%) of 12 of the ganglia from the opposite side reactivated virus after explanation and culture of latently infected trigeminal ganglia. The RNA was transcribed from the strand opposite to that coding for the immediate-early protein; the signal was neuronally localized, with dense nuclear accumulation accompanied by variable numbers of grains over the cytoplasm. Northern RNA blot analysis showed that a discrete set of poly(A)- PRV transcripts were present in latently infected trigeminal ganglia. Additional in situ nucleic acid hybridization analysis revealed that the 3' limit of the transcriptionally active area was located in a 1.2-kilobase fragment upstream and adjacent to the 5' end of the immediate-early protein RNA, whereas the 5' limit was as much as 4.9 kilobases downstream from the 3' end of this RNA. PRV therefore expresses latent-phase transcripts that, although similar in many respects to latent-phase transcripts reported for other herpesviruses, have some unique properties.
Full text
PDF

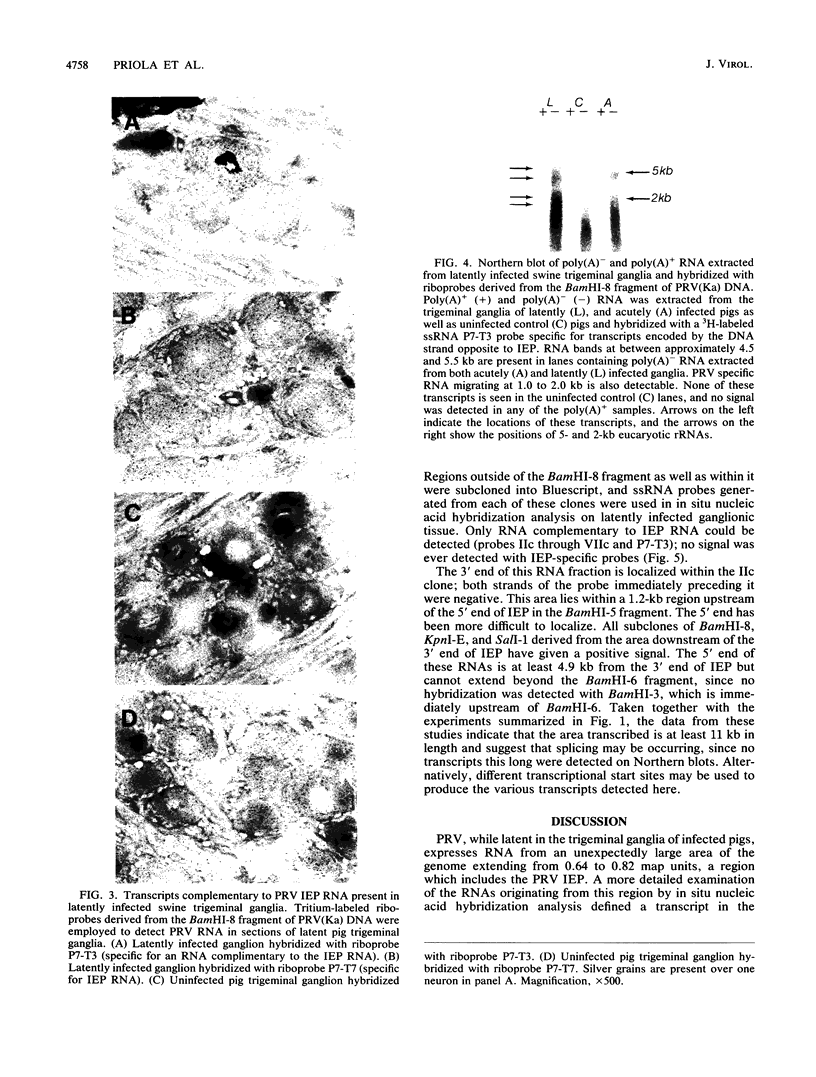


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung A. K. Detection of pseudorabies virus transcripts in trigeminal ganglia of latently infected swine. J Virol. 1989 Jul;63(7):2908–2913. doi: 10.1128/jvi.63.7.2908-2913.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Croen K. D., Ostrove J. M., Dragovic L. J., Straus S. E. Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9773–9777. doi: 10.1073/pnas.85.24.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A. T., Sederati F., Devi-Rao G., Flanagan W. M., Farrell M. J., Stevens J. G., Wagner E. K., Feldman L. T. Identification of the latency-associated transcript promoter by expression of rabbit beta-globin mRNA in mouse sensory nerve ganglia latently infected with a recombinant herpes simplex virus. J Virol. 1989 Sep;63(9):3844–3851. doi: 10.1128/jvi.63.9.3844-3851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y. J., Johnson B., Romanowski E., Araullo-Cruz T. RNA complementary to herpes simplex virus type 1 ICP0 gene demonstrated in neurons of human trigeminal ganglia. J Virol. 1988 May;62(5):1832–1835. doi: 10.1128/jvi.62.5.1832-1835.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutekunst D. E. Latent pseudorabies virus infection in swine detected by RNA-DNA hybridization. Am J Vet Res. 1979 Nov;40(11):1568–1572. [PubMed] [Google Scholar]
- Hill J. M., Sedarati F., Javier R. T., Wagner E. K., Stevens J. G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990 Jan;174(1):117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Ihara S., Feldman L., Watanabe S., Ben-Porat T. Characterization of the immediate-early functions of pseudorabies virus. Virology. 1983 Dec;131(2):437–454. doi: 10.1016/0042-6822(83)90510-x. [DOI] [PubMed] [Google Scholar]
- Krause P. R., Croen K. D., Straus S. E., Ostrove J. M. Detection and preliminary characterization of herpes simplex virus type 1 transcripts in latently infected human trigeminal ganglia. J Virol. 1988 Dec;62(12):4819–4823. doi: 10.1128/jvi.62.12.4819-4823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D. A., Bogard C. L., Kosz-Vnenchak M., Hicks K. A., Coen D. M., Knipe D. M., Schaffer P. A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989 Jul;63(7):2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokensgard J. R., Thawley D. G., Molitor T. W. Pseudorabies virus latency: restricted transcription. Arch Virol. 1990;110(1-2):129–136. doi: 10.1007/BF01310709. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Beam S. L., Mayfield J. E. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J Virol. 1987 Dec;61(12):3827–3831. doi: 10.1128/jvi.61.12.3827-3831.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Hagemoser W. A., Osorio F. A., McAllister H. A. Transcription from the pseudorabies virus genome during latent infection. Brief report. Arch Virol. 1988;98(1-2):99–106. doi: 10.1007/BF01321010. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rziha H. J., Mettenleiter T. C., Ohlinger V., Wittmann G. Herpesvirus (pseudorabies virus) latency in swine: occurrence and physical state of viral DNA in neural tissues. Virology. 1986 Dec;155(2):600–613. doi: 10.1016/0042-6822(86)90220-5. [DOI] [PubMed] [Google Scholar]
- Sabó A., Rajcáni J. Latent pseudorabies virus infection in pigs. Acta Virol. 1976 Jun;20(3):208–214. [PubMed] [Google Scholar]
- Sedarati F., Izumi K. M., Wagner E. K., Stevens J. G. Herpes simplex virus type 1 latency-associated transcription plays no role in establishment or maintenance of a latent infection in murine sensory neurons. J Virol. 1989 Oct;63(10):4455–4458. doi: 10.1128/jvi.63.10.4455-4458.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Lirette R. P., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989 Feb;8(2):505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Haarr L., Porter D. D., Cook M. L., Wagner E. K. Prominence of the herpes simplex virus latency-associated transcript in trigeminal ganglia from seropositive humans. J Infect Dis. 1988 Jul;158(1):117–123. doi: 10.1093/infdis/158.1.117. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989 Sep;53(3):318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Devi-Rao G., Feldman L. T., Dobson A. T., Zhang Y. F., Flanagan W. M., Stevens J. G. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J Virol. 1988 Apr;62(4):1194–1202. doi: 10.1128/jvi.62.4.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Flanagan W. M., Devi-Rao G., Zhang Y. F., Hill J. M., Anderson K. P., Stevens J. G. The herpes simplex virus latency-associated transcript is spliced during the latent phase of infection. J Virol. 1988 Dec;62(12):4577–4585. doi: 10.1128/jvi.62.12.4577-4585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Nesburn A. B., Watson R., Slanina S. M., Ghiasi H. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J Virol. 1988 Nov;62(11):4051–4058. doi: 10.1128/jvi.62.11.4051-4058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby A. J., Blyth W. A., Hill T. J. The effect of DNA hypomethylating agents on the reactivation of herpes simplex virus from latently infected mouse ganglia in vitro. Brief report. Arch Virol. 1987;97(1-2):137–144. doi: 10.1007/BF01310742. [DOI] [PubMed] [Google Scholar]