Abstract
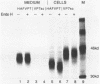
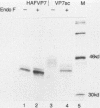
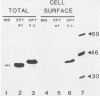
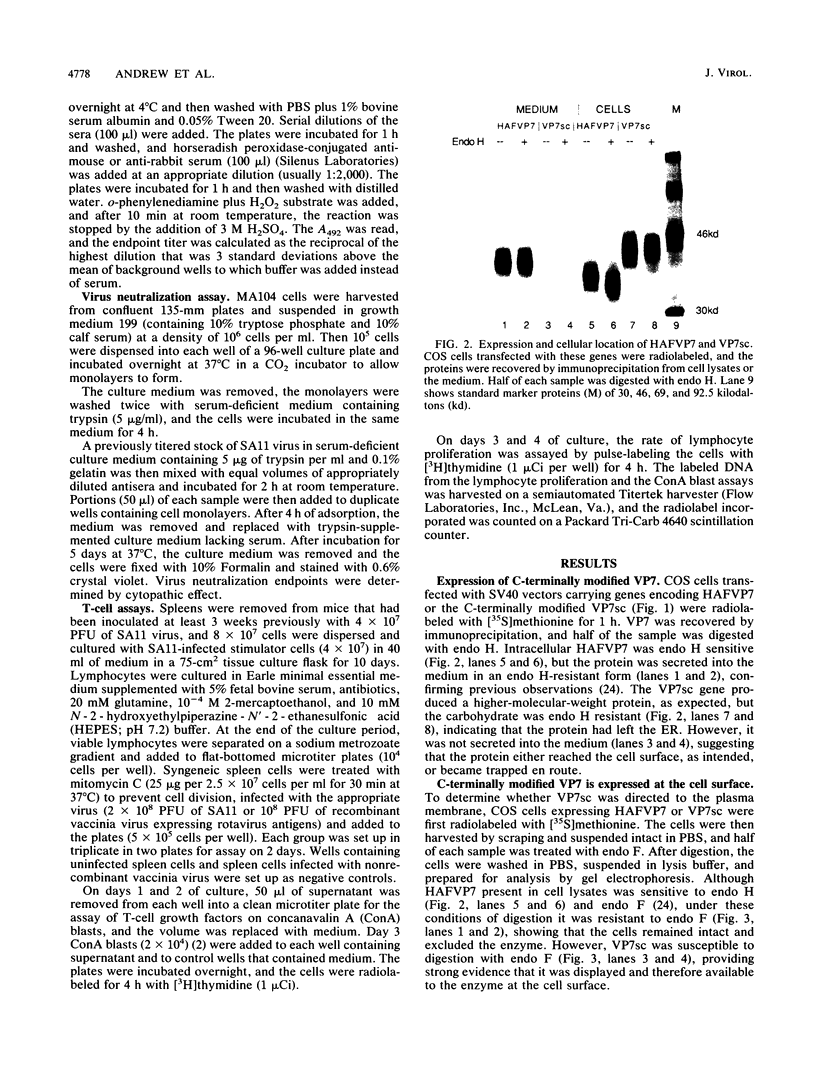
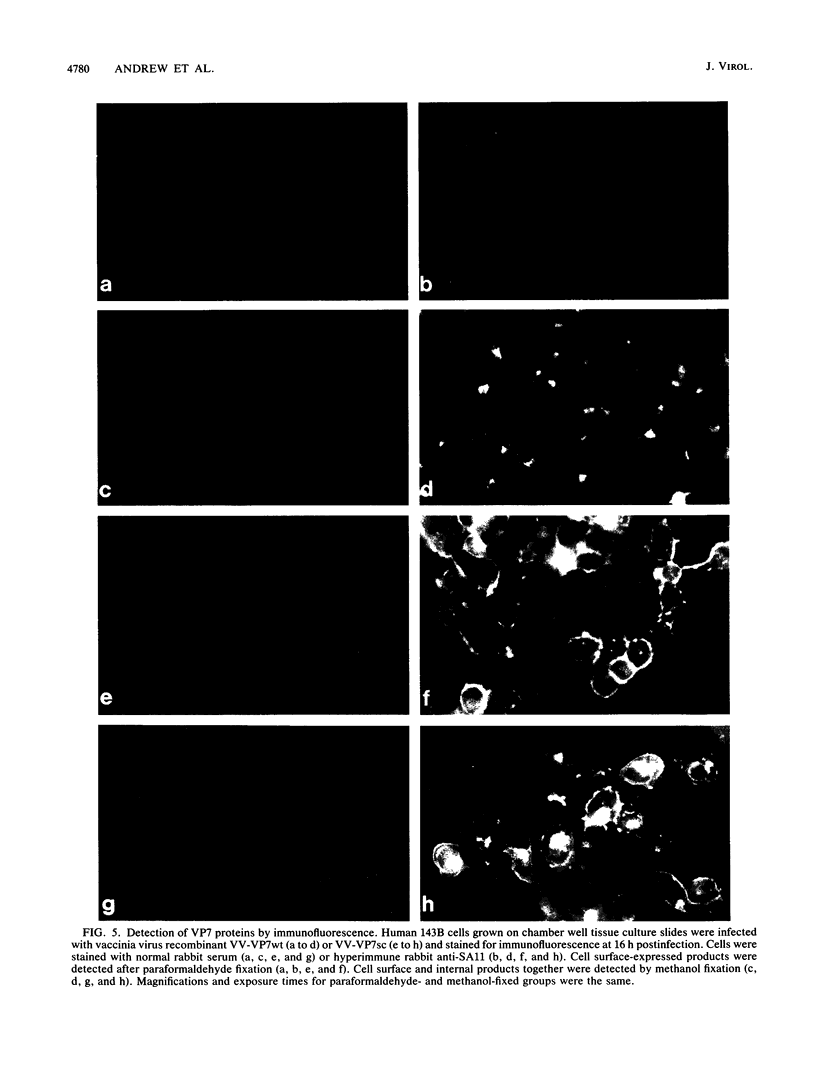
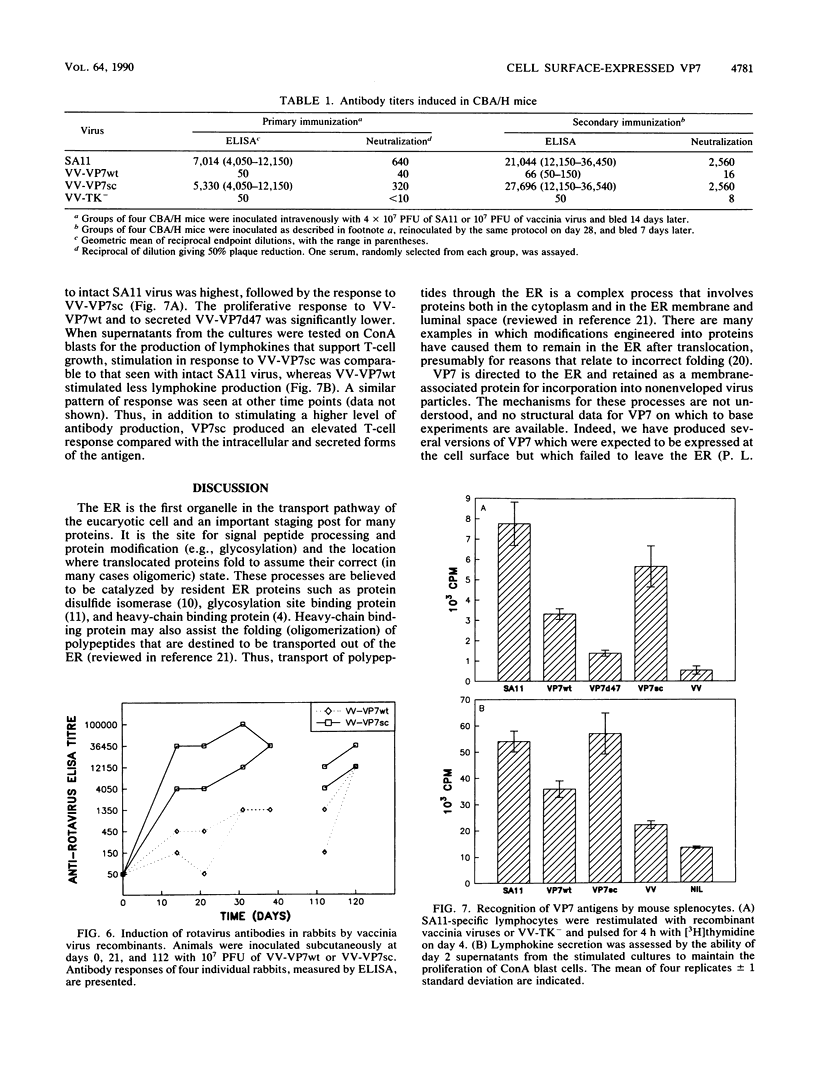
The glycoprotein VP7, the major serotype antigen of rotaviruses, is localized to the endoplasmic reticulum (ER) of the cell, where it is retained as a membrane-associated protein before assembly into mature virus particles. Wild-type VP7 expressed by a recombinant vaccinia virus was also located internally and was poorly antigenic. Using recombinant techniques, a correctly processed, secreted form of VP7 (S.C. Stirzaker and G.W. Both, Cell 56:741-747, 1989) was modified by addition to its C terminus of the membrane anchor and cytoplasmic domains from the influenza virus hemagglutinin. The hybrid protein was directed to the surface of cells, where it was anchored in the plasma membrane. When expressed in mice and rabbits by a recombinant vaccinia virus, the surface-anchored antigen stimulated a level of rotavirus-specific antibodies that was greater than 100-fold above the level induced by wild-type VP7. T-cell responses to the novel antigen were also elevated in comparison with the wild-type, intracellular protein. Cell surface anchoring may provide a strategy to increase the immunogenicity of intracellular antigens from other parasites and viruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew M. E., Boyle D. B., Coupar B. E., Whitfeld P. L., Both G. W., Bellamy A. R. Vaccinia virus recombinants expressing the SA11 rotavirus VP7 glycoprotein gene induce serotype-specific neutralizing antibodies. J Virol. 1987 Apr;61(4):1054–1060. doi: 10.1128/jvi.61.4.1054-1060.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew M. E., Churilla A. M., Malek T. R., Braciale V. L., Braciale T. J. Activation of virus specific CTL clones: antigen-dependent regulation of interleukin 2 receptor expression. J Immunol. 1985 Feb;134(2):920–925. [PubMed] [Google Scholar]
- Blancou J., Kieny M. P., Lathe R., Lecocq J. P., Pastoret P. P., Soulebot J. P., Desmettre P. Oral vaccination of the fox against rabies using a live recombinant vaccinia virus. Nature. 1986 Jul 24;322(6077):373–375. doi: 10.1038/322373a0. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos T. J., Davis A. R., Nayak D. P. NH2-terminal hydrophobic region of influenza virus neuraminidase provides the signal function in translocation. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2327–2331. doi: 10.1073/pnas.81.8.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Mattick J. S., Bellamy A. R. Serotype-specific glycoprotein of simian 11 rotavirus: coding assignment and gene sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3091–3095. doi: 10.1073/pnas.80.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E., Both G. W. Multiple-cloning-site plasmids for the rapid construction of recombinant poxviruses. Gene. 1985;35(1-2):169–177. doi: 10.1016/0378-1119(85)90169-6. [DOI] [PubMed] [Google Scholar]
- Esposito J. J., Murphy F. A. Infectious recombinant vectored virus vaccines. Adv Vet Sci Comp Med. 1989;33:195–247. doi: 10.1016/b978-0-12-039233-9.50010-x. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha-Habib M., Noiva R., Kaplan H. A., Lennarz W. J. Glycosylation site binding protein, a component of oligosaccharyl transferase, is highly similar to three other 57 kd luminal proteins of the ER. Cell. 1988 Sep 23;54(7):1053–1060. doi: 10.1016/0092-8674(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Langford C. J., Edwards S. J., Smith G. L., Mitchell G. F., Moss B., Kemp D. J., Anders R. F. Anchoring a secreted plasmodium antigen on the surface of recombinant vaccinia virus-infected cells increases its immunogenicity. Mol Cell Biol. 1986 Sep;6(9):3191–3199. doi: 10.1128/mcb.6.9.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanski J. L., Pala P., Corradin G., Jordan B. R., Cerottini J. C. H-2-restricted cytolytic T cells specific for HLA can recognize a synthetic HLA peptide. Nature. 1986 Dec 11;324(6097):578–579. doi: 10.1038/324578a0. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–324. doi: 10.1146/annurev.iy.05.040187.001513. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes cross-react with target cells infected with different rotavirus serotypes. J Virol. 1988 Jan;62(1):127–131. doi: 10.1128/jvi.62.1.127-131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
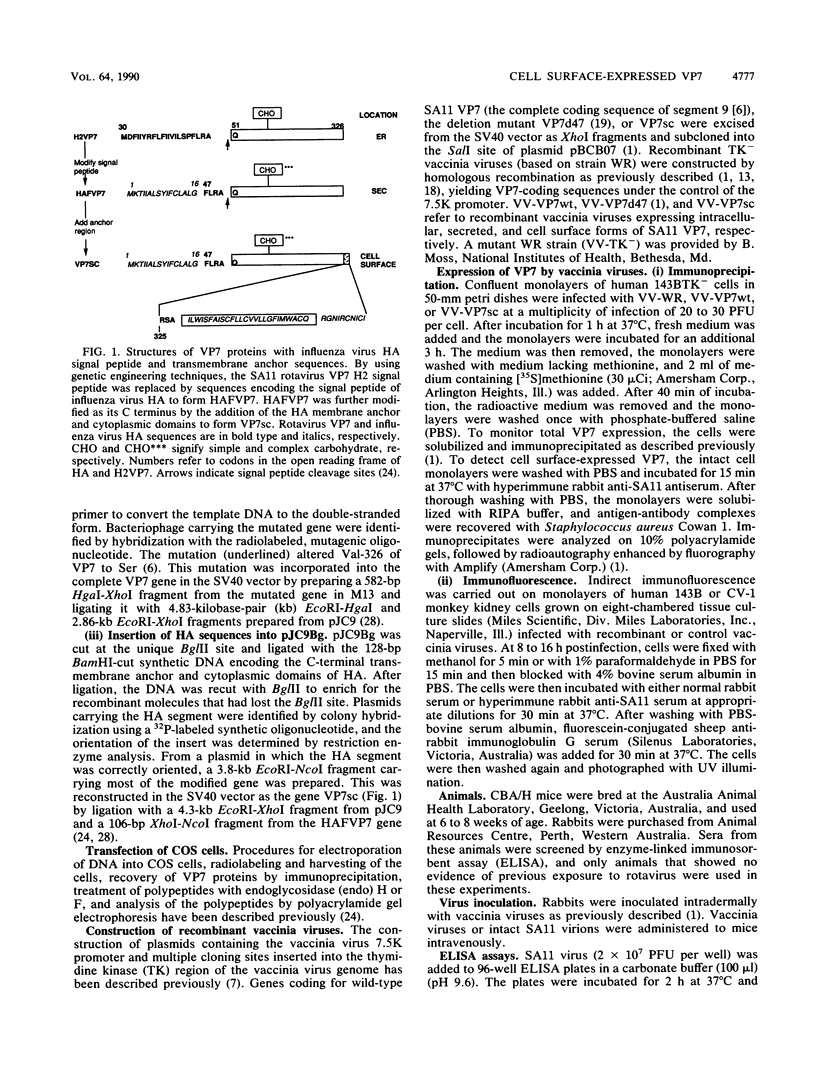
- Poruchynsky M. S., Tyndall C., Both G. W., Sato F., Bellamy A. R., Atkinson P. H. Deletions into an NH2-terminal hydrophobic domain result in secretion of rotavirus VP7, a resident endoplasmic reticulum membrane glycoprotein. J Cell Biol. 1985 Dec;101(6):2199–2209. doi: 10.1083/jcb.101.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Godson G. N., Nussenzweig V., Nussenzweig R. S., Barnwell J., Moss B. Plasmodium knowlesi sporozoite antigen: expression by infectious recombinant vaccinia virus. Science. 1984 Apr 27;224(4647):397–399. doi: 10.1126/science.6200932. [DOI] [PubMed] [Google Scholar]
- Stirzaker S. C., Both G. W. The signal peptide of the rotavirus glycoprotein VP7 is essential for its retention in the ER as an integral membrane protein. Cell. 1989 Mar 10;56(5):741–747. doi: 10.1016/0092-8674(89)90677-6. [DOI] [PubMed] [Google Scholar]
- Stirzaker S. C., Whitfeld P. L., Christie D. L., Bellamy A. R., Both G. W. Processing of rotavirus glycoprotein VP7: implications for the retention of the protein in the endoplasmic reticulum. J Cell Biol. 1987 Dec;105(6 Pt 2):2897–2903. doi: 10.1083/jcb.105.6.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Bastin J., Gould K., Brownlee G. G. Cytotoxic T lymphocytes recognize influenza haemagglutinin that lacks a signal sequence. Nature. 1986 Dec 11;324(6097):575–577. doi: 10.1038/324575a0. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Whitfeld P. L., Tyndall C., Stirzaker S. C., Bellamy A. R., Both G. W. Location of sequences within rotavirus SA11 glycoprotein VP7 which direct it to the endoplasmic reticulum. Mol Cell Biol. 1987 Jul;7(7):2491–2497. doi: 10.1128/mcb.7.7.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilma T., Hsu D., Jones L., Owens S., Grubman M., Mebus C., Yamanaka M., Dale B. Protection of cattle against rinderpest with vaccinia virus recombinants expressing the HA or F gene. Science. 1988 Nov 18;242(4881):1058–1061. doi: 10.1126/science.3194758. [DOI] [PubMed] [Google Scholar]