Abstract
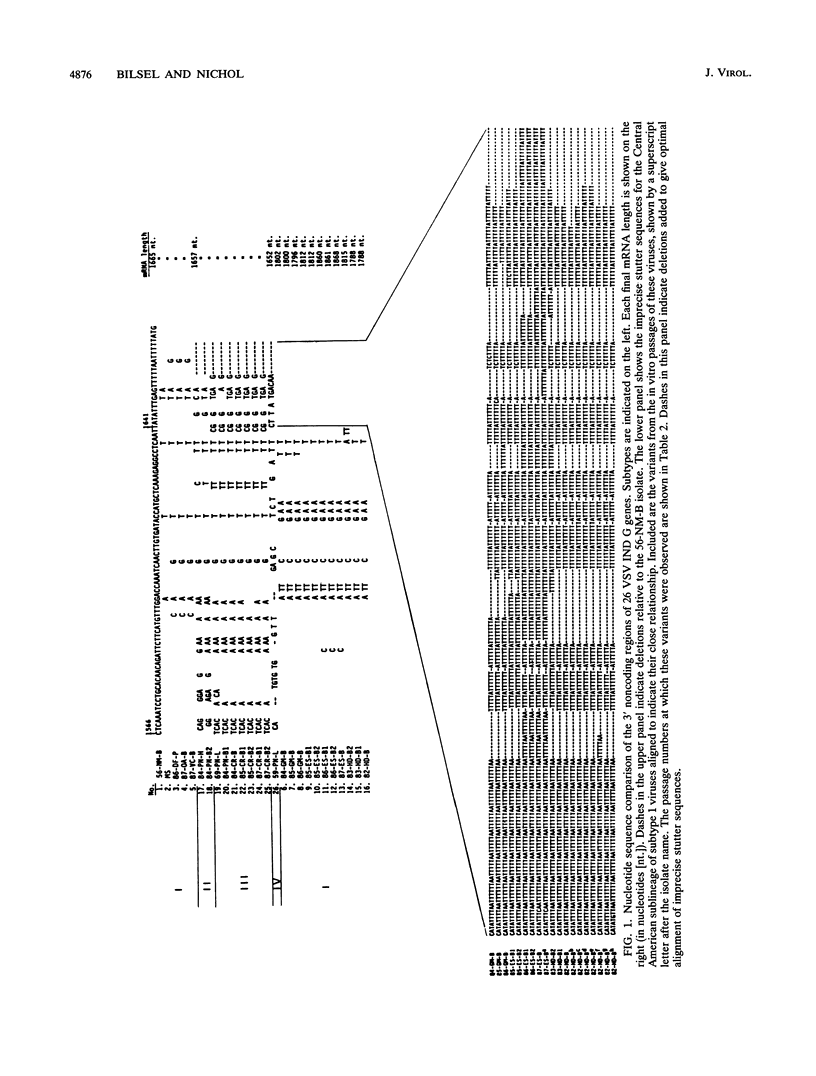
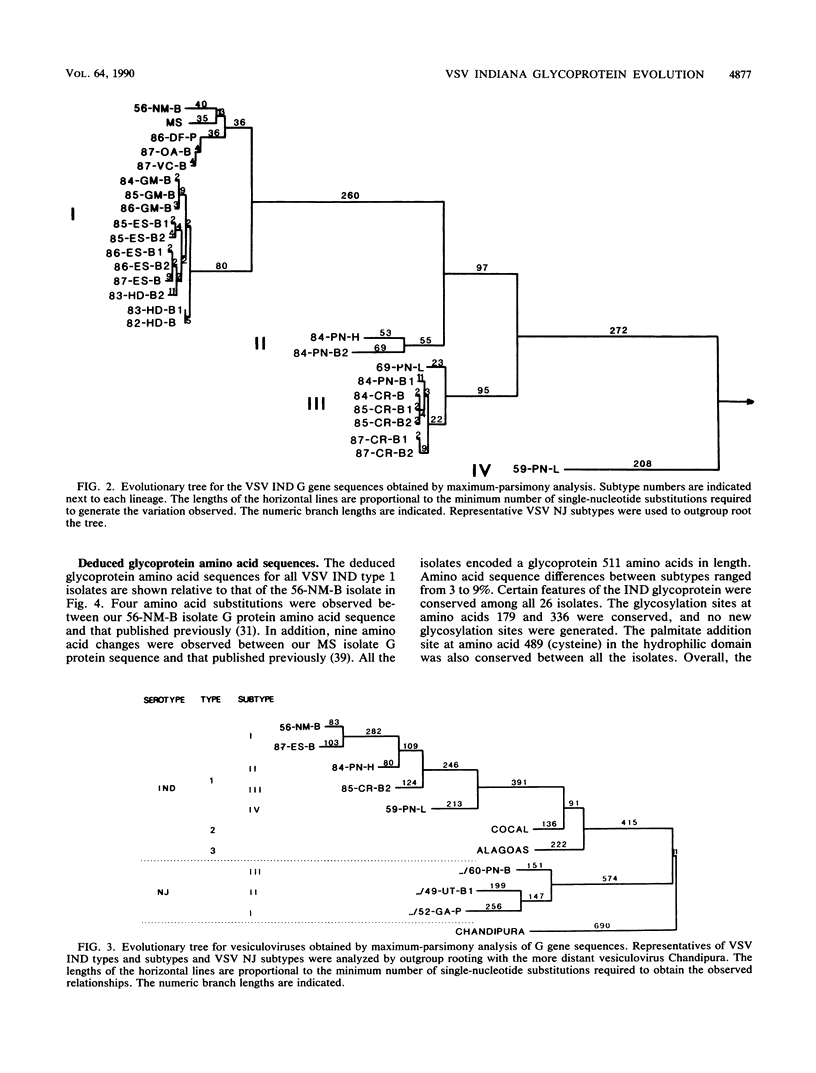
We report the entire glycoprotein (G) gene nucleotide sequences of 26 vesicular stomatitis virus Indiana serotype (VSV IND) type 1 isolates from North and Central America. These sequences are also compared with partial G gene sequences of VSV IND type 2 (Cocal) and type 3 (Alagoas) viruses and the complete G gene sequences of the more distantly related VSV New Jersey (NJ) and Chandipura viruses. Phylogenetic analysis of the G gene sequences by maximum parsimony revealed four major lineages or subtypes within the classical VSV IND (type 1) viruses, each with a distinct geographic distribution. A high degree of VSV genetic diversity was found in Central America, with several virus subtypes of both VSV IND and NJ serotypes existing in this mainly enzootic disease region. Nineteen percent sequence variation but no deletions or insertions were evident within the 5' noncoding and the coding regions of the VSV IND type 1 G genes. In addition to numerous base substitutions, the 3' noncoding regions of these viruses also contained numerous base insertions and deletions. This resulted in striking variation in G gene sizes, with gene lengths ranging from 1,652 to 1,868 nucleotides. As the VSV IND type 1 subtypes have diverged from the common ancestor with the NJ subtypes, their G mRNAs have accumulated more 3' noncoding sequence inserts, ranging up to 303 nucleotides in length. These primarily consist of an imprecise reiteration of the sequence UUUUUAA, apparently generated by a unique polymerase stuttering error. Analysis of the deduced amino acid sequence differences among VSV IND type 1 viruses revealed numerous substitutions within defined antigenic epitopes, suggesting that immune selection may play a role in the evolution of these viruses.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P. N., Rodrigues F. M. Chandipura: a new Arbovirus isolated in India from patients with febrile illness. Indian J Med Res. 1967 Dec;55(12):1295–1305. [PubMed] [Google Scholar]
- Bilsel P. A., Rowe J. E., Fitch W. M., Nichol S. T. Phosphoprotein and nucleocapsid protein evolution of vesicular stomatitis virus New Jersey. J Virol. 1990 Jun;64(6):2498–2504. doi: 10.1128/jvi.64.6.2498-2504.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand C., Palese P. Sequential passage of influenza virus in embryonated eggs or tissue culture: emergence of mutants. Virology. 1980 Dec;107(2):424–433. doi: 10.1016/0042-6822(80)90309-8. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Brown F. Serological relationships between different strains of vesicular stomatis virus. J Gen Virol. 1972 Sep;16(3):391–398. doi: 10.1099/0022-1317-16-3-391. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Bishop D. H., Kang C. Y., Coffin J., Schnitzlein W. M., Reichmann M. E., Shope R. E. Oligonucleotide fingerprints of RNA species obtained from rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J Virol. 1977 Jul;23(1):152–166. doi: 10.1128/jvi.23.1.152-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer J. A., Tesh R. B., Modi G. B., Corn J. L., Nettles V. F. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am J Trop Med Hyg. 1990 May;42(5):483–490. doi: 10.4269/ajtmh.1990.42.483. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione C. J., Rose J. K. Nucleotide sequence of a cDNA clone encoding the entire glycoprotein from the New Jersey serotype of vesicular stomatitis virus. J Virol. 1983 Apr;46(1):162–169. doi: 10.1128/jvi.46.1.162-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. P. The natural history of vesicular stomatitis. Bacteriol Rev. 1952 Sep;16(3):179–204. doi: 10.1128/br.16.3.179-204.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Grabau E. A., Jones C. L., Semler B. L. Evolution of multiple genome mutations during long-term persistent infection by vesicular stomatitis virus. Cell. 1979 Mar;16(3):495–504. doi: 10.1016/0092-8674(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Hunt D. M., Smith E. F., Buckley D. W. Aberrant polyadenylation by a vesicular stomatitis virus mutant is due to an altered L protein. J Virol. 1984 Nov;52(2):515–521. doi: 10.1128/jvi.52.2.515-521.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONKERS A. H., SHOPE R. E., AITKEN T. H., SPENCE L. COCAL VIRUS, A NEW AGENT IN TRINIDAD RELATED TO VESICULAR STOMATITIS VIRUS, TYPE INDIANA. Am J Vet Res. 1964 Jan;25:236–242. [PubMed] [Google Scholar]
- Keil W., Wagner R. R. Epitope mapping by deletion mutants and chimeras of two vesicular stomatitis virus glycoprotein genes expressed by a vaccinia virus vector. Virology. 1989 Jun;170(2):392–407. doi: 10.1016/0042-6822(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Luo L. H., Li Y., Snyder R. M., Wagner R. R. Point mutations in glycoprotein gene of vesicular stomatitis virus (New Jersey serotype) selected by resistance to neutralization by epitope-specific monoclonal antibodies. Virology. 1988 Apr;163(2):341–348. doi: 10.1016/0042-6822(88)90274-7. [DOI] [PubMed] [Google Scholar]
- Luo L. Z., Li Y., Snyder R. M., Wagner R. R. Spontaneous mutations leading to antigenic variations in the glycoproteins of vesicular stomatitis virus field isolates. Virology. 1990 Jan;174(1):70–78. doi: 10.1016/0042-6822(90)90055-v. [DOI] [PubMed] [Google Scholar]
- Masters P. S., Banerjee A. K. Sequences of Chandipura virus N and NS genes: evidence for high mutability of the NS gene within vesiculoviruses. Virology. 1987 Apr;157(2):298–306. doi: 10.1016/0042-6822(87)90272-8. [DOI] [PubMed] [Google Scholar]
- Masters P. S., Bhella R. S., Butcher M., Patel B., Ghosh H. P., Banerjee A. K. Structure and expression of the glycoprotein gene of Chandipura virus. Virology. 1989 Jul;171(1):285–290. doi: 10.1016/0042-6822(89)90540-0. [DOI] [PubMed] [Google Scholar]
- Nichol S. T. Genetic diversity of enzootic isolates of vesicular stomatitis virus New Jersey. J Virol. 1988 Feb;62(2):572–579. doi: 10.1128/jvi.62.2.572-579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol S. T., Holland J. J. Genome RNA terminus conservation and diversity among vesiculoviruses. J Virol. 1987 Jan;61(1):200–205. doi: 10.1128/jvi.61.1.200-205.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol S. T. Molecular epizootiology and evolution of vesicular stomatitis virus New Jersey. J Virol. 1987 Apr;61(4):1029–1036. doi: 10.1128/jvi.61.4.1029-1036.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol S. T., O'Hara P. J., Holland J. J., Perrault J. Structure and origin of a novel class of defective interfering particle of vesicular stomatitis virus. Nucleic Acids Res. 1984 Mar 26;12(6):2775–2790. doi: 10.1093/nar/12.6.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol S. T., Rowe J. E., Fitch W. M. Glycoprotein evolution of vesicular stomatitis virus New Jersey. Virology. 1989 Feb;168(2):281–291. doi: 10.1016/0042-6822(89)90268-7. [DOI] [PubMed] [Google Scholar]
- O'Hara P. J., Horodyski F. M., Nichol S. T., Holland J. J. Vesicular stomatitis virus mutants resistant to defective-interfering particles accumulate stable 5'-terminal and fewer 3'-terminal mutations in a stepwise manner. J Virol. 1984 Mar;49(3):793–798. doi: 10.1128/jvi.49.3.793-798.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz E., Moreno N., Peralta P. H., Tesh R. B. A human case of encephalitis associated with vesicular stomatitis virus (Indiana serotype) infection. Am J Trop Med Hyg. 1988 Sep;39(3):312–314. doi: 10.4269/ajtmh.1988.39.312. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands K., Grabau E., Spindler K., Jones C., Semler B., Holland J. Virus protein changes and RNA termini alterations evolving during persistent infection. Cell. 1980 Apr;19(4):871–880. doi: 10.1016/0092-8674(80)90078-1. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Horodyski F. M., Holland J. J. High multiplicities of infection favor rapid and random evolution of vesicular stomatitis virus. Virology. 1982 May;119(1):96–108. doi: 10.1016/0042-6822(82)90068-x. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., de la Torre J. C., Meier E., Holland J. J. Extreme heterogeneity in populations of vesicular stomatitis virus. J Virol. 1989 May;63(5):2072–2080. doi: 10.1128/jvi.63.5.2072-2080.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh R. B., Boshell J., Modi G. B., Morales A., Young D. G., Corredor A., Ferro de Carrasquilla C., de Rodriguez C., Walters L. L., Gaitan M. O. Natural infection of humans, animals, and phlebotomine sand flies with the Alagoas serotype of vesicular stomatitis virus in Colombia. Am J Trop Med Hyg. 1987 May;36(3):653–661. doi: 10.4269/ajtmh.1987.36.653. [DOI] [PubMed] [Google Scholar]
- Tesh R. B., Chaniotis B. N., Johnson K. M. Vesicular stomatitis virus (Indiana serotype): transovarial transmission by phlebotomine sandlies. Science. 1972 Mar 31;175(4029):1477–1479. doi: 10.1126/science.175.4029.1477. [DOI] [PubMed] [Google Scholar]
- Tesh R. B., Travassos Da Rosa A. P., Travassos Da Rosa J. S. Antigenic relationship among rhabdoviruses infecting terrestrial vertebrates. J Gen Virol. 1983 Jan;64(Pt 1):169–176. doi: 10.1099/0022-1317-64-1-169. [DOI] [PubMed] [Google Scholar]
- Travassos da Rosa A. P., Tesh R. B., Travassos da Rosa J. F., Herve J. P., Main A. J., Jr Carajas and Maraba viruses, two new vesiculoviruses isolated from phlebotomine sand flies in Brazil. Am J Trop Med Hyg. 1984 Sep;33(5):999–1006. doi: 10.4269/ajtmh.1984.33.999. [DOI] [PubMed] [Google Scholar]
- Vandepol S. B., Lefrancois L., Holland J. J. Sequences of the major antibody binding epitopes of the Indiana serotype of vesicular stomatitis virus. Virology. 1986 Jan 30;148(2):312–325. doi: 10.1016/0042-6822(86)90328-4. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Youngner J. S., Keene J. D. Base mutations in the terminal noncoding regions of the genome of vesicular stomatitis virus isolated from persistent infections of L cells. Virology. 1985 Jan 30;140(2):249–256. doi: 10.1016/0042-6822(85)90363-0. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Jones E. V., Kelly M., Frielle D. W. Generation and amplification of temperature-sensitive mutants during serial undiluted passages of vesicular stomatitis virus. Virology. 1981 Jan 15;108(1):87–97. doi: 10.1016/0042-6822(81)90529-8. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Preble O. T., Jones E. V. Persistent infection of L cells with vesicular stomatitis virus: evolution of virus populations. J Virol. 1978 Oct;28(1):6–12. doi: 10.1128/jvi.28.1.6-13.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


