Abstract
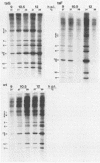
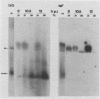
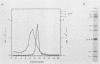
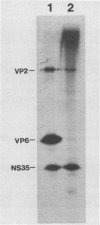
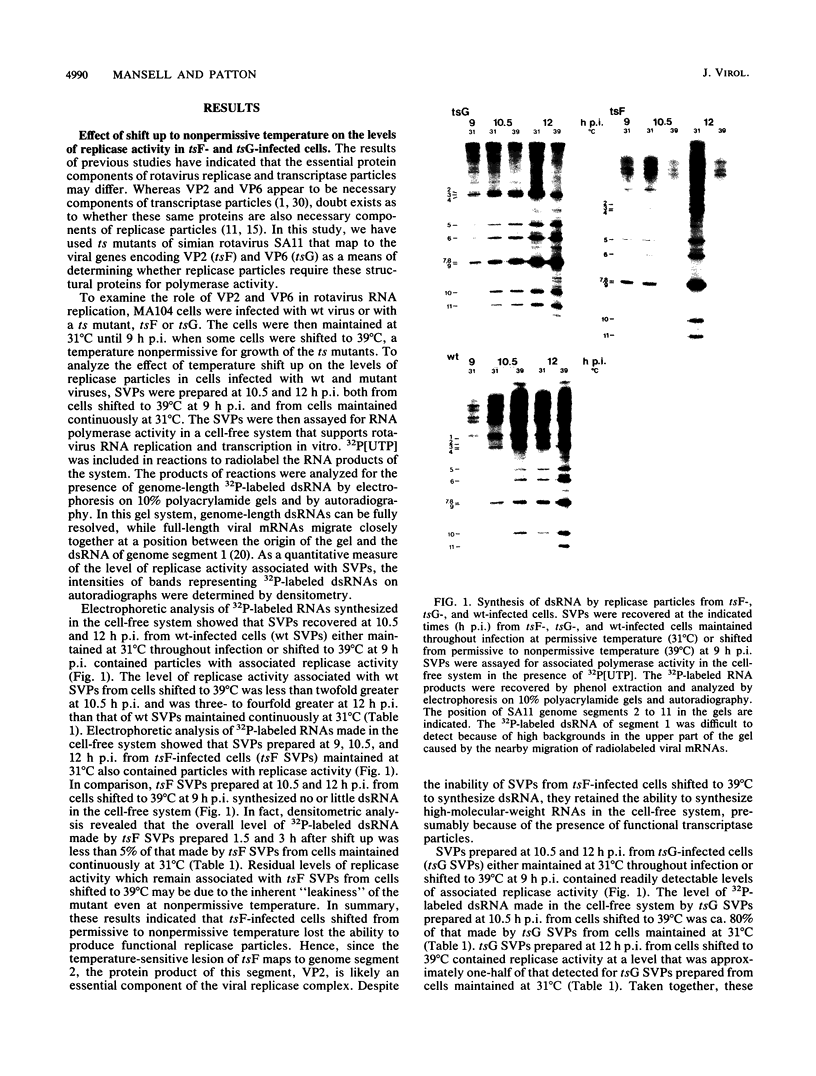
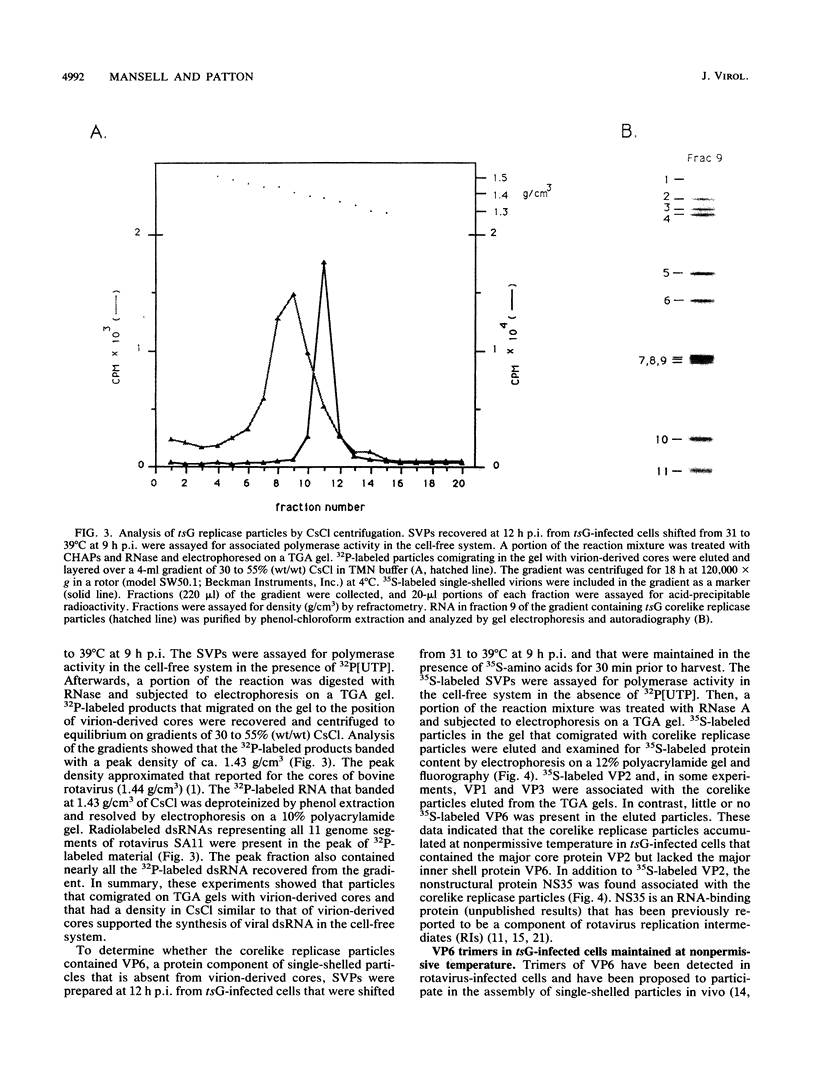
Temperature-sensitive mutants of simian rotavirus SA11 were previously developed and organized into 10 of a possible 11 recombination groups on the basis of genome reassortment studies. Two of these mutants, tsF and tsG, map to genes encoding VP2 (segment 2) and VP6 (segment 6), respectively. To gain insight into the role of these proteins in genome replication, MA104 cells were infected with tsF or tsG and then maintained at permissive temperature (31 degrees C) until 9 h postinfection, when some cells were shifted to nonpermissive temperature (39 degrees C). Subviral particles (SVPs) were recovered from the infected cells at 10.5 and 12 h postinfection and assayed for associated replicase activity in a cell-free system shown previously to support rotavirus genome replication in vitro. The results showed that the level of replicase activity associated with tsF SVPs from cells shifted to nonpermissive temperature was ca. 20-fold less than that associated with tsF SVPs from cells maintained at permissive temperature. In contrast, the level of replicase activity associated with tsG SVPs from cells maintained at nonpermissive temperature was only slightly less (twofold or less) than that associated with tsG SVPs from cells maintained at permissive temperature. Analysis of the structure of replicase particles from tsG-infected cells shifted to nonpermissive temperature showed that they were similar in size and density to virion-derived core particles and contained the major core protein VP2 but lacked the major inner shell protein VP6. Taken together, these data indicate that VP2, but not VP6, is an essential component of enzymatically active replicase particles.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bican P., Cohen J., Charpilienne A., Scherrer R. Purification and characterization of bovine rotavirus cores. J Virol. 1982 Sep;43(3):1113–1117. doi: 10.1128/jvi.43.3.1113-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. F., Holmes K. V. RNA-binding proteins of bovine rotavirus. J Virol. 1986 May;58(2):561–568. doi: 10.1128/jvi.58.2.561-568.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M. L. Human viral gastroenteritis. Clin Microbiol Rev. 1989 Jan;2(1):51–89. doi: 10.1128/cmr.2.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Charpilienne A., Chilmonczyk S., Estes M. K. Nucleotide sequence of bovine rotavirus gene 1 and expression of the gene product in baculovirus. Virology. 1989 Jul;171(1):131–140. doi: 10.1016/0042-6822(89)90519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Ericson B. L., Graham D. Y., Mason B. B., Estes M. K. Identification, synthesis, and modifications of simian rotavirus SA11 polypeptides in infected cells. J Virol. 1982 Jun;42(3):825–839. doi: 10.1128/jvi.42.3.825-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Crawford S. E., Penaranda M. E., Petrie B. L., Burns J. W., Chan W. K., Ericson B., Smith G. E., Summers M. D. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987 May;61(5):1488–1494. doi: 10.1128/jvi.61.5.1488-1494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Mason B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981 Sep;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara N., Nishikawa K., Gorziglia M., Kapikian A. Z. Nucleotide sequence of gene segment 1 of a porcine rotavirus strain. Virology. 1989 Dec;173(2):743–749. doi: 10.1016/0042-6822(89)90590-4. [DOI] [PubMed] [Google Scholar]
- Gallegos C. O., Patton J. T. Characterization of rotavirus replication intermediates: a model for the assembly of single-shelled particles. Virology. 1989 Oct;172(2):616–627. doi: 10.1016/0042-6822(89)90204-3. [DOI] [PubMed] [Google Scholar]
- Gombold J. L., Estes M. K., Ramig R. F. Assignment of simian rotavirus SA11 temperature-sensitive mutant groups B and E to genome segments. Virology. 1985 May;143(1):309–320. doi: 10.1016/0042-6822(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Gombold J. L., Ramig R. F. Assignment of simian rotavirus SA11 temperature-sensitive mutant groups A, C, F, and G to genome segments. Virology. 1987 Dec;161(2):463–473. doi: 10.1016/0042-6822(87)90140-1. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Larrea C., Liprandi F., Esparza J. Biochemical evidence for the oligomeric (possibly trimeric) structure of the major inner capsid polypeptide (45K) of rotaviruses. J Gen Virol. 1985 Sep;66(Pt 9):1889–1900. doi: 10.1099/0022-1317-66-9-1889. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Liu M., Estes M. K. Nucleotide sequence of the simian rotavirus SA11 genome segment 3. Nucleic Acids Res. 1989 Oct 11;17(19):7991–7991. doi: 10.1093/nar/17.19.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Offit P. A., Estes M. K. Identification of the simian rotavirus SA11 genome segment 3 product. Virology. 1988 Mar;163(1):26–32. doi: 10.1016/0042-6822(88)90230-9. [DOI] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. T., Gallegos C. O. Rotavirus RNA replication: single-stranded RNA extends from the replicase particle. J Gen Virol. 1990 May;71(Pt 5):1087–1094. doi: 10.1099/0022-1317-71-5-1087. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Gallegos C. O. Structure and protein composition of the rotavirus replicase particle. Virology. 1988 Oct;166(2):358–365. doi: 10.1016/0042-6822(88)90506-5. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Stacy-Phipps S. Electrophoretic separation of the plus and minus strands of rotavirus SA11 double-stranded RNAs. J Virol Methods. 1986 Jun;13(3):185–190. doi: 10.1016/0166-0934(86)90012-1. [DOI] [PubMed] [Google Scholar]
- Patton J. T. Synthesis of simian rotavirus SA11 double-stranded RNA in a cell-free system. Virus Res. 1986 Dec;6(3):217–233. doi: 10.1016/0168-1702(86)90071-7. [DOI] [PubMed] [Google Scholar]
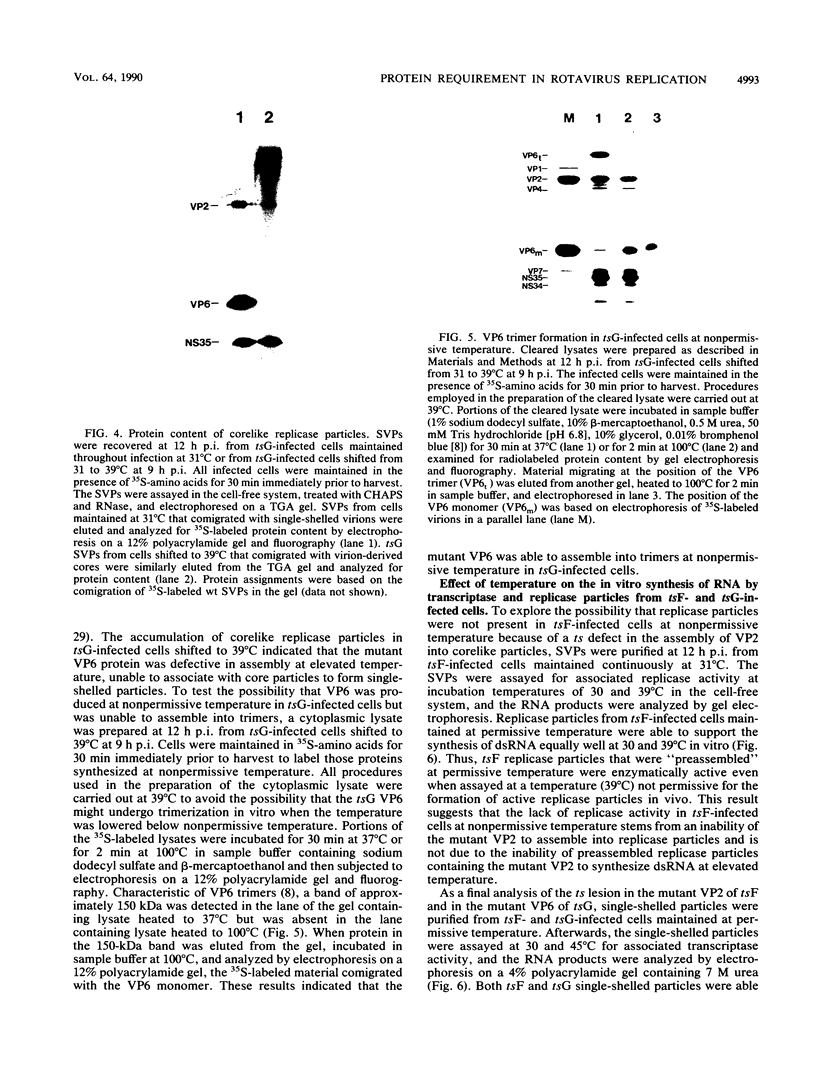
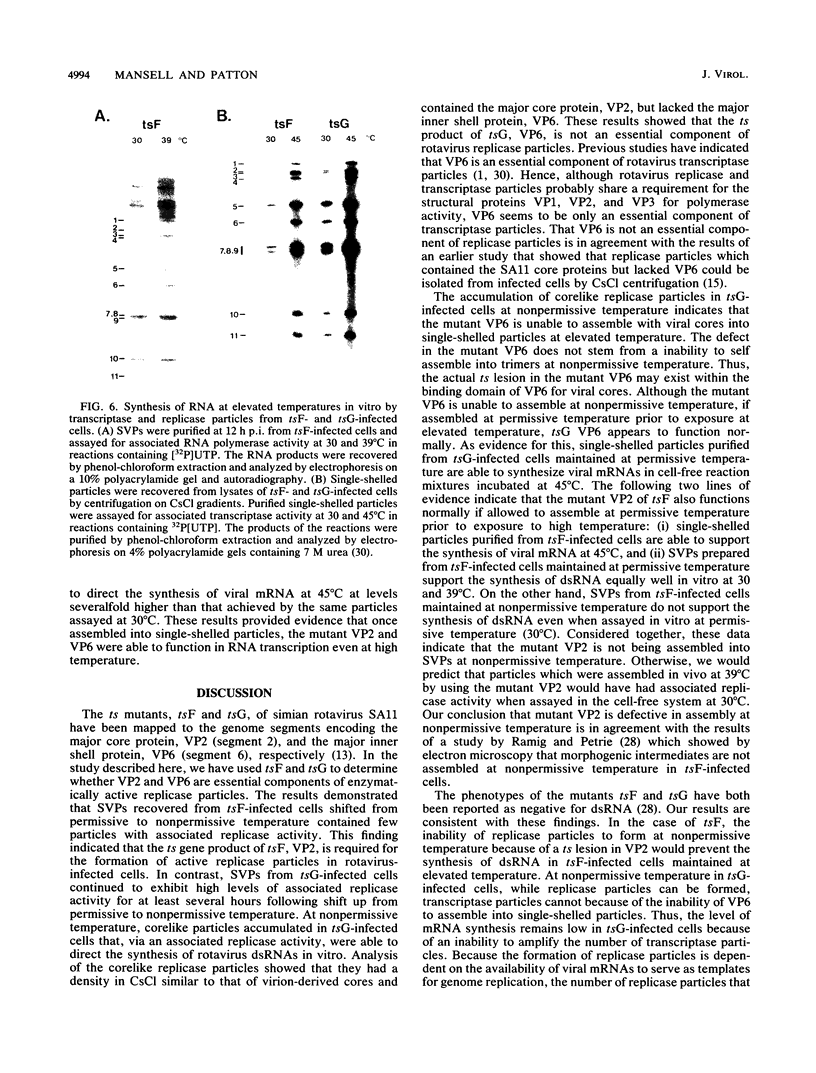
- Petrie B. L., Graham D. Y., Estes M. K. Identification of rotavirus particle types. Intervirology. 1981;16(1):20–28. doi: 10.1159/000149243. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Wang G. J., Clerx J. P., Chiu W. Three-dimensional structure of rotavirus. J Mol Biol. 1988 Jan 20;199(2):269–275. doi: 10.1016/0022-2836(88)90313-0. [DOI] [PubMed] [Google Scholar]
- Ramig R. F. Isolation and genetic characterization of temperature-sensitive mutants of simian rotavirus SA11. Virology. 1982 Jul 15;120(1):93–105. doi: 10.1016/0042-6822(82)90009-5. [DOI] [PubMed] [Google Scholar]
- Ramig R. F. Isolation and genetic characterization of temperature-sensitive mutants that define five additional recombination groups in simian rotavirus SA11. Virology. 1983 Oct 30;130(2):464–473. doi: 10.1016/0042-6822(83)90100-9. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Petrie B. L. Characterization of temperature-sensitive mutants of simian rotavirus SA11: protein synthesis and morphogenesis. J Virol. 1984 Mar;49(3):665–673. doi: 10.1128/jvi.49.3.665-673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Ready K. F., Frenchick P. J., Babiuk L. A. Biochemical evidence for the oligomeric arrangement of bovine rotavirus nucleocapsid protein and its possible significance in the immunogenicity of this protein. J Gen Virol. 1987 Jan;68(Pt 1):123–133. doi: 10.1099/0022-1317-68-1-123. [DOI] [PubMed] [Google Scholar]
- Sandino A. M., Jashes M., Faúndez G., Spencer E. Role of the inner protein capsid on in vitro human rotavirus transcription. J Virol. 1986 Nov;60(2):797–802. doi: 10.1128/jvi.60.2.797-802.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]