Abstract
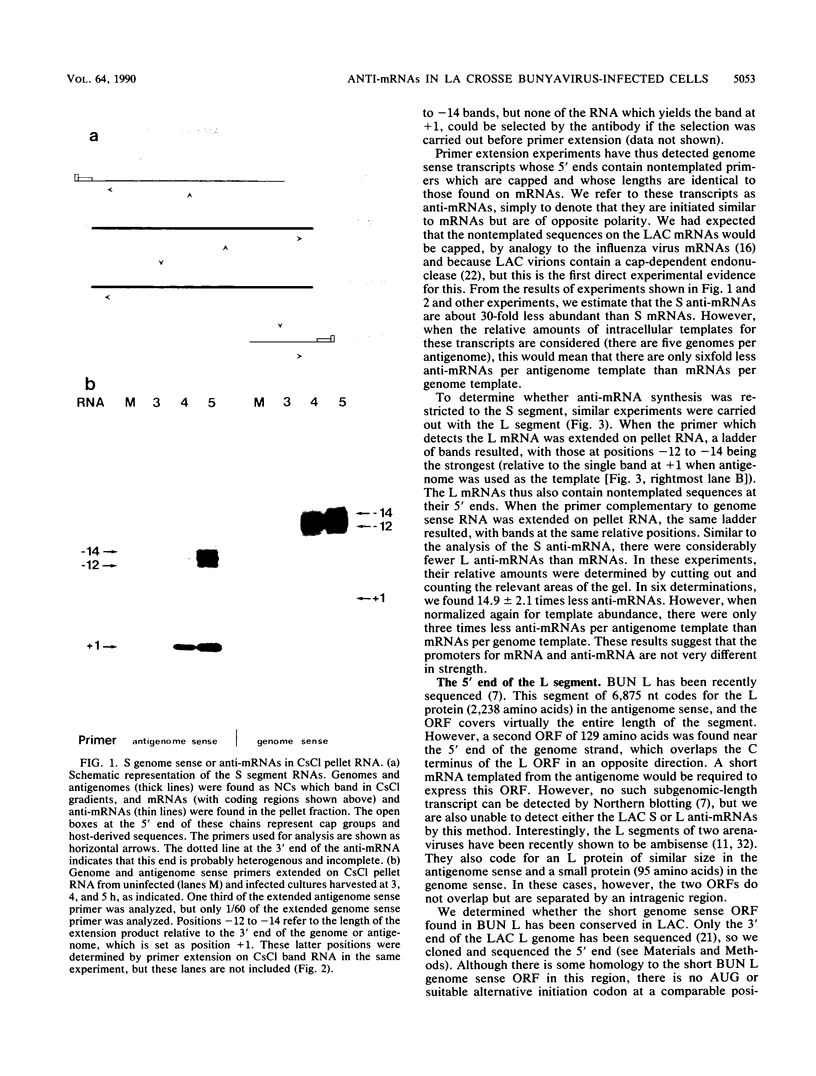
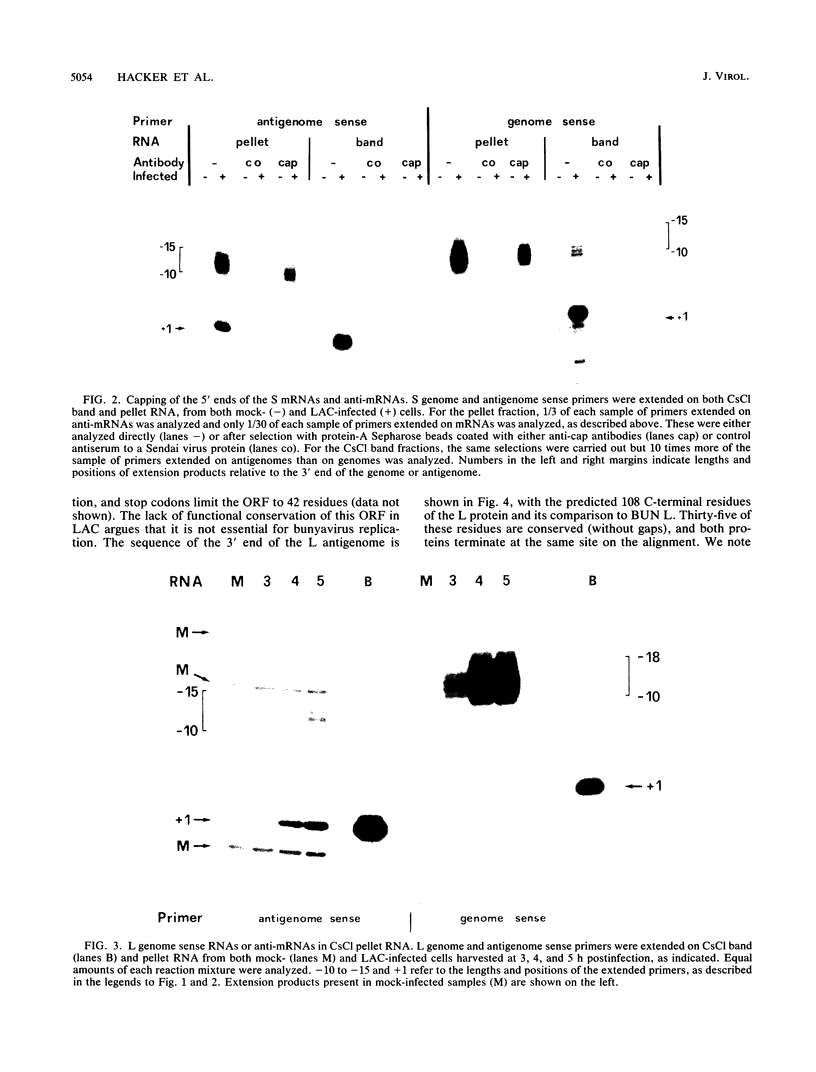
Unlike some members of the family Bunyaviridae which contain ambisense genomes, all La Crosse virus reading frames are translated from antigenome sense mRNAs. Nevertheless, La Crosse virus genome sense mRNAs or anti-mRNAs are initiated from antigenome templates. These are characterized by the same range of capped, nontemplated sequences at their 5' ends as mRNAs, but their 3' ends are presumed to be heterogenous, as they were not seen on RNA blots. The anti-mRNAs are estimated to be 15 to 30 times less abundant than mRNAs, but remarkably, this ratio is similar to that of functional genome sense mRNAs made from other bona fide ambisense segments. A role for these anti-mRNAs during infection is unclear.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auperin D. D., Romanowski V., Galinski M., Bishop D. H. Sequencing studies of pichinde arenavirus S RNA indicate a novel coding strategy, an ambisense viral S RNA. J Virol. 1984 Dec;52(3):897–904. doi: 10.1128/jvi.52.3.897-904.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988 Dec 23;55(6):1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Bishop D. H. Ambisense RNA genomes of arenaviruses and phleboviruses. Adv Virus Res. 1986;31:1–51. doi: 10.1016/S0065-3527(08)60261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Gay M. E., Matsuoko Y. Nonviral heterogeneous sequences are present at the 5' ends of one species of snowshoe hare bunyavirus S complementary RNA. Nucleic Acids Res. 1983 Sep 24;11(18):6409–6418. doi: 10.1093/nar/11.18.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabradilla C. D., Jr, Holloway B. P., Obijeski J. F. Molecular cloning and sequencing of the La Crosse virus S RNA. Virology. 1983 Jul 30;128(2):463–468. doi: 10.1016/0042-6822(83)90271-4. [DOI] [PubMed] [Google Scholar]
- Case C. C., Roels S. M., Jensen P. D., Lee J., Kleckner N., Simons R. W. The unusual stability of the IS10 anti-sense RNA is critical for its function and is determined by the structure of its stem-domain. EMBO J. 1989 Dec 20;8(13):4297–4305. doi: 10.1002/j.1460-2075.1989.tb08616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. M. Molecular biology of the Bunyaviridae. J Gen Virol. 1990 Mar;71(Pt 3):501–522. doi: 10.1099/0022-1317-71-3-501. [DOI] [PubMed] [Google Scholar]
- Elliott R. M. Nucleotide sequence analysis of the large (L) genomic RNA segment of Bunyamwera virus, the prototype of the family Bunyaviridae. Virology. 1989 Dec;173(2):426–436. doi: 10.1016/0042-6822(89)90555-2. [DOI] [PubMed] [Google Scholar]
- Grady L. J., Sanders M. L., Campbell W. P. The sequence of the M RNA of an isolate of La Crosse virus. J Gen Virol. 1987 Dec;68(Pt 12):3057–3071. doi: 10.1099/0022-1317-68-12-3057. [DOI] [PubMed] [Google Scholar]
- Hacker D., Raju R., Kolakofsky D. La Crosse virus nucleocapsid protein controls its own synthesis in mosquito cells by encapsidating its mRNA. J Virol. 1989 Dec;63(12):5166–5174. doi: 10.1128/jvi.63.12.5166-5174.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara T., Akashi H., Bishop D. H. Novel coding strategy (ambisense genomic RNA) revealed by sequence analyses of Punta Toro Phlebovirus S RNA. Virology. 1984 Jul 30;136(2):293–306. doi: 10.1016/0042-6822(84)90166-1. [DOI] [PubMed] [Google Scholar]
- Ihara T., Matsuura Y., Bishop D. H. Analyses of the mRNA transcription processes of Punta Toro phlebovirus (Bunyaviridae). Virology. 1985 Dec;147(2):317–325. doi: 10.1016/0042-6822(85)90134-5. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. W. An antisense mRNA directs the covalent modification of the transcript encoding fibroblast growth factor in Xenopus oocytes. Cell. 1989 Nov 17;59(4):687–696. doi: 10.1016/0092-8674(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Bellocq C., Raju R. The translational requirement for La Crosse virus S-mRNA synthesis. Cold Spring Harb Symp Quant Biol. 1987;52:373–379. doi: 10.1101/sqb.1987.052.01.043. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Priming of influenza viral RNA transcription by capped heterologous RNAs. Curr Top Microbiol Immunol. 1981;93:125–149. doi: 10.1007/978-3-642-68123-3_6. [DOI] [PubMed] [Google Scholar]
- Marriott A. C., Ward V. K., Nuttall P. A. The S RNA segment of Sandfly Fever Sicilian virus: evidence for an ambisense genome. Virology. 1989 Apr;169(2):341–345. doi: 10.1016/0042-6822(89)90159-1. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Chen Y. M., Foss K., Schneider J. Association of transfer RNA acceptor identity with a helical irregularity. Science. 1988 Dec 23;242(4886):1681–1684. doi: 10.1126/science.2462282. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Bishop D. H., Palmer E. L., Murphy F. A. Segmented genome and nucleocapsid of La Crosse virus. J Virol. 1976 Dec;20(3):664–675. doi: 10.1128/jvi.20.3.664-675.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., McCauley J., Skehel J. J. Nucleotide sequences at the terminal of La Crosse virus RNAs. Nucleic Acids Res. 1980 Jun 11;8(11):2431–2438. doi: 10.1093/nar/8.11.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L., Holloway B., Kolakofsky D. La Crosse virions contain a primer-stimulated RNA polymerase and a methylated cap-dependent endonuclease. J Virol. 1984 Oct;52(1):215–222. doi: 10.1128/jvi.52.1.215-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L., Kolakofsky D. Characterization of La Crosse virus small-genome transcripts. J Virol. 1984 Mar;49(3):680–685. doi: 10.1128/jvi.49.3.680-685.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R. F., von Bonsdorff C. H. Ribonucleoproteins of Uukuniemi virus are circular. J Virol. 1975 Feb;15(2):386–392. doi: 10.1128/jvi.15.2.386-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. La Crosse virus infection of mammalian cells induces mRNA instability. J Virol. 1988 Jan;62(1):27–32. doi: 10.1128/jvi.62.1.27-32.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. The ends of La Crosse virus genome and antigenome RNAs within nucleocapsids are base paired. J Virol. 1989 Jan;63(1):122–128. doi: 10.1128/jvi.63.1.122-128.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Translational requirement of La Crosse virus S-mRNA synthesis: in vivo studies. J Virol. 1987 Jan;61(1):96–103. doi: 10.1128/jvi.61.1.96-103.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Unusual transcripts in La Crosse virus-infected cells and the site for nucleocapsid assembly. J Virol. 1987 Mar;61(3):667–672. doi: 10.1128/jvi.61.3.667-672.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Raju L., Kolakofsky D. The translational requirement for complete La Crosse virus mRNA synthesis is cell-type dependent. J Virol. 1989 Dec;63(12):5159–5165. doi: 10.1128/jvi.63.12.5159-5165.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier C., Patterson J., Kolakofsky D. La Crosse virus small genome mRNA is made in the cytoplasm. J Virol. 1986 May;58(2):647–650. doi: 10.1128/jvi.58.2.647-650.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier C., Raju R., Kolakofsky D. LaCrosse virus gene expression in mammalian and mosquito cells. Virology. 1988 Aug;165(2):539–548. doi: 10.1016/0042-6822(88)90598-3. [DOI] [PubMed] [Google Scholar]
- Salvato M. S., Shimomaye E. M. The completed sequence of lymphocytic choriomeningitis virus reveals a unique RNA structure and a gene for a zinc finger protein. Virology. 1989 Nov;173(1):1–10. doi: 10.1016/0042-6822(89)90216-x. [DOI] [PubMed] [Google Scholar]
- Samso A., Bouloy M., Hannoun C. Présence de ribonucléoprotéines circulaires dans le virus Lumbo (Bunyavirus) C R Acad Sci Hebd Seances Acad Sci D. 1975 Feb 10;280(6):779–782. [PubMed] [Google Scholar]
- Schwer B., Stunnenberg H. G. Vaccinia virus late transcripts generated in vitro have a poly(A) head. EMBO J. 1988 Apr;7(4):1183–1190. doi: 10.1002/j.1460-2075.1988.tb02929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J. F., Hellman U., Pettersson R. F. Uukuniemi virus S RNA segment: ambisense coding strategy, packaging of complementary strands into virions, and homology to members of the genus Phlebovirus. J Virol. 1990 Jan;64(1):247–255. doi: 10.1128/jvi.64.1.247-255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. R., Puglisi J. D., Tinoco I., Jr RNA folding: pseudoknots, loops and bulges. Bioessays. 1989 Oct;11(4):100–106. doi: 10.1002/bies.950110406. [DOI] [PubMed] [Google Scholar]